Abstract
Aims/hypothesis
26RFa (pyroglutamilated RFamide peptide [QRFP]) is a biologically active peptide that regulates glucose homeostasis by acting as an incretin and by increasing insulin sensitivity at the periphery. 26RFa is also produced by a neuronal population localised in the hypothalamus. In this study we investigated whether 26RFa neurons are involved in the hypothalamic regulation of glucose homeostasis.
Methods
26Rfa+/+, 26Rfa–/– and insulin-deficient male C57Bl/6J mice were used in this study. Mice received an acute intracerebroventricular (i.c.v.) injection of 26RFa, insulin or the 26RFa receptor (GPR103) antagonist 25e and were subjected to IPGTTs, insulin tolerance tests, acute glucose-stimulated insulin secretion tests and pyruvate tolerance tests (PTTs). Secretion of 26RFa by hypothalamic explants after incubation with glucose, leptin or insulin was assessed. Expression and quantification of the genes encoding 26RFa, agouti-related protein, the insulin receptor and GPR103 were evaluated by quantitative reverse transcription PCR and RNAscope in situ hybridisation.
Results
Our data indicate that i.c.v.-injected 26RFa induces a robust antihyperglycaemic effect associated with an increase in insulin production by the pancreatic islets. In addition, we found that insulin strongly stimulates 26Rfa expression and secretion by the hypothalamus. RNAscope experiments revealed that neurons expressing 26Rfa are mainly localised in the lateral hypothalamic area, that they co-express the gene encoding the insulin receptor and that insulin induces the expression of 26Rfa in these neurons. Concurrently, the central antihyperglycaemic effect of insulin is abolished in the presence of a GPR103 antagonist and in 26RFa-deficient mice. Finally, our data indicate that the hypothalamic 26RFa neurons are not involved in the central inhibitory effect of insulin on hepatic glucose production, but mediate the central effects of the hormone on its own peripheral production.
Conclusion/interpretation
We have identified a novel mechanism in the hypothalamic regulation of glucose homeostasis, the 26RFa/GPR103 system, and we provide evidence that this neuronal peptidergic system is a key relay for the central regulation of glucose metabolism by insulin.
Graphical abstract

Similar content being viewed by others

Introduction
It has been known since the 19th century that the brain, and more specifically the hypothalamus, is involved in the control of glucose homeostasis and diabetes pathogenesis [1, 2]. Evidence has been provided that the brain accounts for 50% of overall glucose disposal and can normalise glycaemia in rodent models of diabetes [3, 4]. From these findings a new model has emerged in which the control of glucose homeostasis results from complex and highly coordinated interactions between the brain and the pancreatic islets. The crucial role of the brain in the control of glucose metabolism is supported by observations that the injection of glucose, leptin or insulin into discrete hypothalamic areas, including the arcuate nucleus (Arc), the ventromedial hypothalamic nucleus (VMH) and the paraventricular nucleus, lowers blood glucose levels and increases insulin sensitivity in the liver [2, 5, 6] and that central insulin decreases hepatic glucose production (HGP) [7]. Similar effects are achieved by restoring functional insulin receptors in specific hypothalamic nuclei that otherwise lack them [8]. Conversely, deletion of insulin receptors or their downstream signalling intermediates from defined hypothalamic neurons induces glucose intolerance and systemic insulin resistance [9, 10]. More specifically, it was found that the central effects of insulin on glucose homeostasis are mediated only in part by the agouti-related protein (AgRP) neurons of the Arc and not by the proopiomelanocortin (POMC) neurons [10, 11]. These observations suggest that insulin targets other neuropeptidergic systems in the hypothalamus to exert its central action on glucose homeostasis and highlight that our knowledge on the molecular identity of the hypothalamic neuronal populations relaying the central action of insulin remains fragmentary.
In this context, the neuropeptidergic 26RFa/GPR103 system is of particular interest. 26RFa (also referred to as pyroglutamilated RFamide peptide [QRFP]) is a hypothalamic neuropeptide that was discovered concurrently by us and others [12,13,14]. It has been identified as the cognate ligand of the human orphan G protein-coupled receptor GPR103 [13,14,15,16,17]. Neuroanatomical observations revealed that 26RFa- and GPR103-expressing neurons are primarily localised in two hypothalamic nuclei involved in the control of feeding behaviour, that is, the lateral hypothalamic area (LHA) and the VMH [12, 16, 18, 19]. Indeed, intracerebroventricular (i.c.v.) administration of 26RFa stimulates food intake [12, 16, 20, 21], and the neuropeptide exerts its orexigenic activity by modulating the neuropeptide Y/POMC system in the Arc [21]. More recently, the involvement of the 26RFa/GPR103 neuropeptidergic system in the control of glucose homeostasis at the periphery was reported. We and others found that 26Rfa (also known as Qrfp) and Gpr103 (also known as Qrfpr) are strongly expressed by beta cells of the pancreatic islets [22,23,24] and that the neuropeptide prevents cell death and apoptosis of beta cells [22]. We also showed that 26Rfa is abundantly expressed all along the gut and that i.p. administration of the neuropeptide attenuates glucose-induced hyperglycaemia by increasing plasma insulin through a direct insulinotropic effect on the pancreatic beta cells and by increasing insulin sensitivity [23, 24]. Finally, we reported that an oral glucose challenge induces a massive secretion of 26RFa by the gut into the blood, strongly suggesting that this neuropeptide regulates blood or plasma glucose by acting as an incretin [23]. This incretin effect of 26RFa has been confirmed by the observations that administration of a GPR103 antagonist reduces the global glucose-induced incretin effect and also decreases insulin sensitivity [25] and that 26RFa-deficient mice show impaired regulation of glucose homeostasis [26].
Altogether, these observations prompted us to investigate whether the hypothalamic 26RFa/GPR103 system is involved in the central regulation of glucose homeostasis and may be a target of insulin.
Methods
Animals
26RFa+/+ (wild-type mice), 26RFa–/– (mutant mice) and insulin-deficient male C57Bl/6J mice (2–4 months old), weighing 22–25 g, were used in this study. 26RFa–/– (iCre knock-in) mice were obtained from T. Sakurai (International Institute for Integrative Sleep Medecine, Tsukuba, Ibaraki, Japan). The mutant mice were generated by homologous recombination in embryonic stem cells of 129SvJ mice, which were then implanted in C57 blastocysts using standard procedures. The targeting vector was constructed by replacing the entire coding region of the prepro-26Rfa sequence in exon 2 of the 26Rfa gene with the iCre sequence and pgk-Neo cassette [26]. Insulin deficiency was induced by a single i.p. injection of streptozotocin (150 mg/kg) 7 days before performing the experiments. Insulin deficiency was assessed by measurement of glycaemia and insulinaemia 7 days post streptozotocin administration. Animals that exhibited high basal glycaemia or did not respond to the glucose challenge with a significant hyperglycaemic peak were excluded. Mice were housed with free access to a standard diet (UAR, Villemoisson-sur-Orge, France) and tap water. They were kept in a ventilated room at a temperature of 22 ± 1°C under a 12 h light/12 h dark cycle (light on between 07:00 and 19:00).
All experimental procedures were approved by the Normandy Regional Ethics Committee (authorisation: APAFIS no. 11752-2017100916177319) and were carried out in accordance with Council Directive 86/609/EEC of 24 November 1986.
Animal surgery
26Rfa+/+, 26Rfa–/– and insulin-deficient mice were anaesthetised with isoflurane and placed in a stereotaxic frame. A stainless steel 26 gauge guide cannula (Phymep, Paris, France) was inserted into the right lateral ventricle (0.8 mm lateral to the bregma and 2.2 mm ventral to the dura mater). A stainless steel dummy small cap for 26 gauge cannulae (Phymep) was inserted to prevent occlusion of the guide cannula and leakage of cerebrospinal fluid. The cannula placement was secured with dental acrylic cement. Correct cannula positioning was confirmed by histological examination after Trypan Blue injection. The i.c.v. injections were performed using a Hamilton syringe (outer diameter 0.5 mm; Hamilton, Reno, NV, USA) attached to polyethylene tubing. The injections were performed under anaesthesia, which was induced by i.p. administration of diazepam (5 mg/kg) and ketamine (100 mg/kg).
Hypothalamic explants
Hypothalami from freshly euthanised 26Rfa+/+ mice were quickly removed and transferred into individual tubes containing 3 ml of artificial cerebrospinal fluid medium composed of 20 mmol/l NaHCO3, 126 mmol/l NaCl, 0.09 mmol/l Na2HPO4, 6 mmol/l KCl, 1.4 mmol/l CaCl2, 0.09 mmol/l MgSO4, 2 mmol/l glucose, 0.18 mg/ml ascorbic acid and 100 g/ml aprotinin. The tubes were maintained at 37°C in 95% O2 and 5% CO2 under constant stirring. After incubation with glucose (0.5 or 5 mmol/l), leptin (10 or 100 nmol/l) or insulin (10 or 100 nmol/l) for 4 h, the culture media were collected for 26RFa assay and the hypothalami were stored at −80°C until gene quantification by qRT-PCR.
Quantitative reverse transcription PCR
Total RNA from hypothalamic explants of 26Rfa+/+ mice was isolated as previously described [25]. Relative expression of Gpr103, 26Rfa and Insr genes was quantified by quantitative reverse transcription PCR (qRT-PCR) using appropriate primers (see electronic supplementary material [ESM] Table 1). Actb, the gene encoding β-actin, was used as an internal control for normalisation. PCR was carried out using a Gene Expression Master Mix 2X assay (Applied Biosystems, Courtaboeuf, France) in an ABI Prism 7900HT Fast Real-time PCR System (Applied Biosystems). The purity of the PCR products was assessed using dissociation curves. The amount of target cDNA was calculated using the Ct method and expressed by means of the \( {2}^{-\Delta \Delta {\mathrm{C}}_{\mathrm{t}}} \) method.
Blood glucose and insulin measurements in mice
For measurements of basal blood or plasma glucose and insulinaemia, 26Rfa+/+, 26Rfa–/– and insulin-deficient mice were fasted for 6 h or 12 h before the test with free access to water. For IPGTTs, mice were fasted for 16 h with free access to water and then injected intraperitoneally with glucose (2 g/kg). For insulin tolerance tests, mice were fasted for 6 h with free access to water and then injected intraperitoneally with human insulin (0.75 units/kg body weight; Eli Lilly, Neuilly-sur-Seine, France). For PTTs, mice were fasted for 16 h with free access to water and then injected intraperitoneally with sodium pyruvate (2 g/kg; Sigma Aldrich, Saint-Quentin Fallavier, France). Test substances, including 26RFa (3 μg), the GPR103 antagonist 25e (10−4 mol/l) and insulin (10 mU), were dissolved in HEPES buffer and injected intracerebroventricularly in a volume of 2 μl. Plasma glucose concentrations were measured in tail vein samples at various times using an Accu-Chek Performa glucometer (Roche Diagnostic, Saint-Égrève, France). Plasma insulin concentrations (in tail vein samples) were determined using the ultrasensitive mouse insulin AlphaLISA detection kit (Perkin Elmer, Villebon-sur-Yvette, France).
26RFa radioimmunoassay
Quantification of 26RFa in culture media was carried out using a specific radioimmunoassay set up in the laboratory (3). Briefly, each culture medium sample was diluted (1:1) in a solution of water/trifluoroacetic acid (TFA) (99.9:0.1; vol./vol.) and pumped at a flow rate of 1.5 ml/min through a Sep-Pak C18 cartridge (Waters, Guyancourt, France). Bound material was eluted with acetonitrile/water/TFA (50:49.9:0.1; vol./vol./vol.) and acetonitrile was evaporated under reduced pressure. Finally, the dried extracts were resuspended in 0.1 mol/l PBS and assayed for 26RFa.
RNAscope in situ hybridisation experiments
26Rfa+/+ and 26Rfa–/– mice were deeply anaesthetised and perfused transcardially with sterile PBS followed by sterile ice-cold phosphate-buffered 4% paraformaldehyde (pH 7.4) 45 min after i.c.v. administration of HEPES buffer or insulin (10 mU). The brains were removed, post-fixed overnight at 4°C in sterile 4% paraformaldehyde and transferred to sterile 15% sucrose (12 h) and then to sterile 30% sucrose (12 h) in 0.1 mol/l PBS (pH 7.4) at 4°C. Finally, 12-μm-thick sections, collected every 40 μm over the whole length of the hypothalamus (15 sections/hypothalamus), were cut on a cryostat and mounted on SuperFrost Plus slides (Thermo Fisher Scientific, Illkirch, France) and subsequently stored at −80°C. Fluorescent in situ hybridisation for the simultaneous detection of 26Rfa, Insr and Agrp transcripts was performed using an RNAscope assay (Advanced Cell Diagnostics [ACD], Rennes, France). The Insr probe targeted the region 7059-8053 (accession number NM_010568.2; ACD, Cat no. 401011-C2). The 26Rfa probe targeted the region 112–1081 (accession number NM_183424.4; ACD, Cat no. 464341-C3). The Agrp probe targeted the region 11–764 (accession number NM_001271806.1; ACD, Cat no. 400711). Negative and positive control probes recognising dihydrodipicolinate reductase and DapB (a bacterial transcript) and PolR2A (C1 channel), PPIB (C2 channel) and UBC (C3 channel), respectively, were processed in parallel with the target probes to ensure tissue RNA integrity and optimal assay performance. In addition, the specificity of the 26Rfa probe was assessed by performing hybridisation of brain sections of 26Rfa–/– mice using the RNAscope procedure; in these mice there was no 26Rfa mRNA signal (ESM Fig. 1). All incubation steps were performed at 40°C using a humidified chamber and a HybEz oven (ACD). On the day of the assay, all the hybridisation steps (i.e. target retrieval, dehydration, probe hybridisation, amplification steps and detection of the probe) were performed according to the online protocol for the RNAscope multifluorescent assay.
Images were captured using a confocal Leica TCS SP-8-X microscope equipped with a 40× objective and a Leica Thunder Imager 3D (DM6 B) (Leica, Paris, France). Maximum intensity projections were made in Fiji/Image J V1.53q (National Institutes of Health, Washington, DC) and images were adjusted for brightness and contrast. Quantitative analysis (number of 26Rfa-expressing neurons and area of expression of 26Rfa labelling) of the hypothalamic sections was performed also using Fiji/Image J V1.53q.
Statistical analysis
For all the experiments the samples were randomised using a simple randomisation method and the experimenters were blind to group assignment. Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software, San Diego, CA). A Kruskal–Wallis test with Dunn’s multiple comparison test or ordinary one-way ANOVA with Sidak’s multiple comparison was used for comparisons between groups. Two-way ANOVA was used for repeated measures. A post hoc comparison using a Bonferroni, Tukey or Sidak test was applied according to the ANOVA results. All data are presented as mean ± SEM. Statistical significance was set at p<0.05.
Results
Effect of central administration of 26RFa on glucose homeostasis in 26Rfa +/+ and insulin-deficient mice
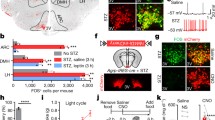
After 6 h or 12 h of fasting, i.c.v. administration of 26RFa did not affect basal plasma glucose levels in 26Rfa+/+ mice during the 90 min period of the test (Fig. 1a, b). By contrast, central injection of 26RFa significantly attenuated (p<0.01) the hyperglycaemia induced by an IPGTT across the whole duration of the IPGTT in 26Rfa+/+ mice (Fig. 1c). Administration of the 26RFa receptor antagonist 25e strongly diminished (p<0.05) the antihyperglycaemic effect of 26RFa in 26Rfa+/+ mice, whereas 25e alone had no effect on plasma glucose levels (Fig. 1d). An acute glucose-stimulated insulin secretion (AGSIS) test revealed that i.c.v. injection of 26RFa significantly potentiates (p<0.01) glucose-induced insulin production in 26Rfa+/+ mice (Fig. 1e). Concurrently, in streptozotocin-treated mice that are unable to produce and secrete insulin, the central antihyperglycaemic effect of 26RFa was totally abolished (Fig. 1f). Finally, 26RFa had no effect on insulin-induced hypoglycaemia during the first 30 min of an insulin tolerance test in 26Rfa+/+ mice, but the peptide attenuated (p<0.05) the hypoglycaemic action of insulin during the last phase of the test (Fig. 1g).
Effect of central administration of 26RFa on glucose homeostasis in 26Rfa+/+ and insulin-deficient mice. All experiments were carried out in 26Rfa+/+ mice other than that described in (f). (a, b) Effect of i.c.v. administration of 26RFa (3 μg) on plasma glucose levels in the basal condition after a 6 h (a) or 12 h (b) fasting period (n = 10–15). (c, d) Effect of i.c.v. administration of 26RFa alone (3 μg) (c) or together with the GPR103 antagonist 25e (10–4 mol/l) (d) on plasma glucose levels during an IPGTT (n = 15). (e) Effect of i.c.v. administration of 26RFa (3 μg) on insulin production during an AGSIS test (n = 11). (f) Effect of i.c.v. administration of 26RFa (3 μg) on plasma glucose levels during an IPGTT in streptozotocin-treated mice (n = 10). (g) Effect of i.c.v. administration of 26RFa (3 μg) on plasma glucose levels during an insulin tolerance test (n = 16). Data are mean ± SEM of four independent experiments for each test, with the AUC shown on a separate graph. T0, time 0. *p<0.05; **p<0.01; ***p<0.001 (26RFa vs vehicle). †p<0.05 (26RFa+25e vs 26RFa alone). ¶p<0.05; ¶¶p<0.01 (26RFa vs 25e)
Effect of glucose, leptin and insulin on 26RFa-producing neurons in 26Rfa +/+ mice
The impact of factors that are well known to regulate glucose homeostasis, such as glucose, leptin and insulin, on hypothalamic 26RFa-producing neurons was investigated using hypothalamic explants. We found that glucose did not significantly alter the secretion of 26RFa and expression of 26Rfa and its receptor Gpr103 by the whole hypothalamus (Fig. 2a–c). Leptin did not alter 26RFa secretion by the hypothalamic explants (Fig. 2d) but stimulated significantly (p<0.05) the expression of the peptide at a dose of 10 nmol/l (Fig. 2e). The expression of Gpr103 was not impaired by leptin (Fig. 2f). Finally, we found that insulin strongly stimulated 26RFa release by the hypothalamic explants in a dose-dependent manner (p<0.01) (Fig. 2g) without modifying the expression of the peptide or its receptor (Fig. 2h, i).
Effect of glucose, leptin and insulin on 26RFa-producing neurons in 26Rfa+/+ mice. (a–i) Effect of various concentrations of glucose, leptin and insulin on the secretion of 26RFa and expression of 26Rfa and Gpr103 by hypothalamic explants. Data are mean ± SEM of four independent experiments (n = 4–11 per condition). *p<0.05; **p<0.01 (vs 0 nmol/l of leptin [e] or 0 nmol/l of insulin [g])
Neuroanatomical interactions between insulin and 26Rfa-expressing neurons in 26Rfa +/+ mice
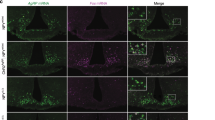
The neuroanatomical interactions between insulin and 26Rfa-expressing neurons were investigated using RNAscope technology. As a first step we mapped the population of 26Rfa-expressing neurons along the whole length of the hypothalamus (Fig. 3). We observed that the great majority of the 26Rfa-expressing neurons were localised in the LHA (Fig. 3d–i). In addition, scattered 26Rfa-expressing neurons were found in the VMH (Fig. 3b–g) and the basal part of the tuber cinereum area (Fig. 3b–d). Concurrently, we mapped the expression of Insr and found that it was widely distributed in all the nuclei of the hypothalamus (Fig. 4a). Co-expression in situ hybridisation experiments revealed that 79% of the 26Rfa-expressing neurons of the hypothalamus expressed Insr (Fig. 4b). Higher magnification observations showed that the Arc exhibited strong Agrp and Insr labelling but was totally devoid of 26Rfa-expressing neurons (Fig. 4c). By contrast, the LHA and to a lesser extent the VMH contained a number of 26Rfa-expressing neurons that mostly co-expressed Insr (Fig. 4c). In a second step, we investigated the impact of i.c.v. administration of insulin during an IPGTT on the expression of 26Rfa and Insr using the qRT-PCR and RNAscope approaches. The qRT-PCR experiment revealed that central injection of insulin increases significantly (p<0.05) the hypothalamic expression of 26Rfa, but does not affect Insr mRNA levels (Fig. 5a). Quantitative analysis performed over the entire length of the hypothalamus using the RNAscope approach revealed that i.c.v. administration of insulin induced an increase in the number of 26Rfa-expressing neurons (p=0.14) (Fig. 5b) and in the area of 26RFa labelling (p=0.12) (Fig. 5c). Representative panoramic views taken during the RNAscope procedure in the anterior part of the hypothalamus illustrate the increase in the number of 26Rfa-expressing neurons in insulin-injected brains vs HEPES injected brains (Fig. 5d, e). Higher magnification photomicrographs (right panels) confirm that, under these two conditions, 26Rfa-expressing neurons co-express Insr (Fig. 5d, e).
Distribution of 26Rfa-expressing neurons in the hypothalami of 26Rfa+/+ mice as determined by RNAscope in situ hybridisation. (a) Schematic parasagittal section of the mouse brain indicating the levels of the sections depicted in (b–i). (b–i) Panoramic views of hypothalamic sections taken from the anterior to the posterior part of the hypothalamus showing that the main population of 26Rfa-expressing neurons (red spots) is localised in the LHA (d–i), with two subpopulations found in the VMH (b–g) and the tuber cinereum area (b–d). Scale bars: 200 μm
Co-expression of 26Rfa with the Insr in the hypothalami of 26Rfa+/+ mice as determined by RNAscope in situ hybridisation. (a) Panoramic section of the anterior part of the hypothalamus showing the wide distribution of Insr (white spots). (b) Quantitative analysis of the number of 26Rfa-expressing neurons that co-express Insr, showing that 79% of the 26RFa neurons express Insr. Data represent mean ± SEM of three independent experiments (n = 5 per condition). (c) Representative photomicrographs of the Arc reveal robust labelling of Agrp (green spots) and Insr (white spots) and the absence of 26Rfa expression. Representative photomicrographs of double labelling in the LHA and the VMH show that 26Rfa (red spots) and Insr (white spots) are co-expressed in the same neurons (arrowheads). However, some 26Rfa-expressing neurons do not express Insr (arrows). Scale bars: (a) 200 μm; (b) 20 μm
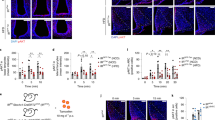
Effect of i.c.v. administration of insulin on the expression of 26Rfa in the hypothalami of 26Rfa+/+ mice as determined by RNAscope in situ hybridisation. (a) Effect of i.c.v. administration of insulin (10 mU) on the hypothalamic expression of 26Rfa and Insr, showing a significant increase in 26Rfa mRNA levels and no effect on Insr mRNA levels. (b, c) Quantitative analysis of the number of 26Rfa-expressing neurons and the area of 26Rfa labelling over the entire length of the hypothalamus, showing an increase in these two complementary variables in insulin-injected brains vs vehicle-injected brains. Data are mean ± SEM of three independent experiments (n = 5–6 per condition). *p<0.05. (d, e) Panoramic views of sections through the anterior part of the hypothalamus of brains injected with the vehicle (d) or insulin (e), showing the occurrence of strongly labelled 26Rfa-expressing neurons (red spots), notably in the LHA of insulin-treated brains. Higher magnification photomicrographs (right panels) reveal that most of the 26Rfa-expressing neurons co-express Insr (white spots; arrowheads). Scale bars: panoramic views, 200 μm; high magnification photomicrographs, 20 μm
Effect of blocking the 26RFa/GPR103 system on insulin hypothalamic signalling using 26Rfa +/+, 26Rfa –/– and insulin-deficient mice
As expected, an IPGTT revealed that i.c.v. administration of insulin induces a robust significant antihyperglycaemic effect in 26Rfa+/+ mice (p<0.05 to p<0.001) (Fig. 6a). Interestingly, 26RFa displayed an antihyperglycaemic profile very similar in terms of amplitude and kinetics to that of insulin (Fig. 6a). Co-administration of the GPR103 antagonist 25e with insulin during an IPGTT resulted in a total blockade of the antihyperglycaemic effect of insulin during the first 60 min of the test in 26Rfa+/+ mice, whereas the administration of 25e alone did not affect the glycaemic profile (Fig. 6b). In addition, an IPGTT performed in mice deficient for 26RFa revealed a loss of the antihyperglycaemic effect of insulin compared with the injection of insulin in wild-type animals (Fig. 6c).
Central interaction between insulin and the hypothalamic 26RFa/GPR103 system in 26Rfa+/+ and 26Rfa–/– mice during an IPGTT. (a) Effect of i.c.v. administration of insulin (10 mU) or 26RFa (3 μg) on plasma glucose levels during an IPGTT in 26Rfa+/+ mice. (b) Effect of i.c.v. administration of insulin (10 mU) alone or with the GPR103 receptor antagonist 25e (10-4 mol/l) on plasma glucose levels during an IPGTT in 26Rfa+/+ mice. (c) Effect of i.c.v. administration of insulin (10 mU) on plasma glucose levels during an IPGTT in 26Rfa+/+ and 26Rfa–/– mice. Data are mean ± SEM of three independent experiments (n=12–16), with the AUC shown on a separate graph. *p<0.05; **p<0.01; ***p<0.001 (insulin vs vehicle). §p<0.05; §§p<0.01; §§§p<0.001 (26RFa vs vehicle [a] or insulin vs 25e [b], or insulin in 26Rfa–/– mice vs insulin in 26Rfa+/+ mice [c]). ¶p<0.05; ¶¶p<0.01 (insulin+25e vs 25e alone). †p<0.05; ††p<0.01; †††p<0.001 (insulin in 26Rfa+/+ mice vs vehicle in 26Rfa–/– mice)
The impact of i.c.v. administration of insulin and 26RFa on HGP during a PTT was also investigated. Central injection of insulin in 26Rfa+/+ mice induced a significant inhibition (from p<0.05 to p<0.01) of HGP that was not blocked by the co-administration of the 26RFa receptor antagonist 25e (Fig. 7a). In contrast to insulin, 26RFa did not alter HGP and the co-administration of the peptide with the GPR103 antagonist did not induce any change in the glycaemic profile in 26Rfa+/+ mice (Fig. 7b). Finally, we found that the inhibitory effect of central insulin on HGP was not altered in 26RFa-mutant mice compared with their wild-type littermates (Fig. 7c).
Central interaction between insulin and the hypothalamic 26RFa/GPR103 system in 26Rfa+/+, 26Rfa–/– and insulin-deficient mice during a PTT, an AGSIS test and an IPGTT. (a) Effect of i.c.v. administration of insulin (10 mU) alone or with the GPR103 receptor antagonist 25e (10–4 mol/l) on plasma glucose levels during a PTT in 26Rfa+/+ mice. (b) Effect of i.c.v. administration of 26RFa (3 μg) alone or with the GPR103 receptor antagonist 25e (10–4 mol/l) on plasma glucose levels during a PTT in 26Rfa+/+ mice. (c) Effect of i.c.v. administration of insulin (10 mU) on plasma glucose levels during a PTT in 26Rfa+/+ and 26Rfa–/– mice. (d) Effect of i.c.v. administration of insulin (10 mU) alone or with the GPR103 receptor antagonist 25e (10–4 mol/l) on plasma insulin levels during an AGSIS test in 26Rfa+/+ and 26Rfa–/– mice. (e) Effect of i.c.v. administration of insulin (10 mU) on plasma glucose levels during an IPGTT in streptozotocin-treated mice. Data are mean ± SEM of three independent experiments (n=9–15), with the AUC shown on a separate graph. *p<0.05; **p<0.01 (insulin vs vehicle in 26Rfa+/+ mice [a] or insulin in 26Rfa–/– mice vs insulin in 26Rfa+/+ mice [d]); §p<0.5; §§p<0.01, §§§p<0.001 (insulin+25e vs insulin alone in 26Rfa+/+ mice)
The effect of central insulin on its own peripheral production by the pancreatic islets during an AGSIS test was also examined in 26Rfa+/+ and 26Rfa–/– mice (Fig. 7d). Our data revealed that the stimulating effect of central insulin on its peripheral production was significantly attenuated by the co-administration of the GPR103 antagonist (from p<0.05 to p<0.001) and in 26RFa-deficient mice (p<0.01). Concurrently, we found that, in streptozotocin-treated mice (in which beta cells are not functional), the robust central antihyperglycaemic effect of insulin observed in Fig. 7a was dramatically impaired during an IPGTT (Fig. 7e).
Discussion
Although it is now recognised that the hypothalamus plays an important role in the global regulation of glucose metabolism in coordination with the pancreatic islets [1, 2], the molecular characterisation of the neuronal circuits involved in the hypothalamic regulation of glucose homeostasis remains fragmentary. Indeed, accumulating evidence supports the view that the AgRP and POMC neuronal systems of the Arc play a pivotal role in the central control of glucose metabolism [10, 11]. However, physiological and genetic approaches indicate that these two peptidergic systems are not sufficient to explain and to understand the hypothalamic regulation of glucose homeostasis [11]. In the present study, we provide evidence that the 26RFa/GPR103 neuropeptidergic system of the hypothalamus plays a key role in the central control of glucose homeostasis.
We found that i.c.v. administration of 26RFa induces a sustained antihyperglycaemic effect that is associated with an increase in insulin production by the pancreatic islets without affecting insulin sensitivity. In addition, we show that this central antihyperglycaemic effect of 26RFa is abolished when a GPR103 antagonist is co-administrated as well as in streptozotocin-treated mice, in which no insulin is produced by the pancreatic islets. Altogether, these findings indicate that 26RFa is involved in the brain regulation of glucose homeostasis and that this central effect of 26RFa is mediated by the activation of its receptor GPR103, leading to stimulation of peripheral insulin production by the pancreatic islets. We previously showed that, at the periphery, 26RFa produced by the gut and the pancreatic islets displays robust antihyperglycaemic activity by acting as an incretin and by triggering insulin sensitivity [23,24,25]. The present study suggests that the global antihyperglycaemic effect of 26RFa is the result of the dual action of the peptide on the pancreatic islets and on the hypothalamus. 26RFa is not the only factor that is able to regulate glycaemia by acting synergically at the peripheral and central levels. For instance, it has been reported that injection of glucose, leptin or insulin within the hypothalamus lowers blood glucose levels and increases insulin sensitivity in the liver [2, 5, 6], and that insulin decreases HGP [7]. It has also been observed that central injection of leptin (at doses too low to have any effect outside the brain) is able to normalise glycaemia in mice models of severe insulin deficiency [4]. Hormones other than leptin can also act in the brain to promote insulin-independent glucose lowering. For instance, the gastrointestinal hormone fibroblast growth factor 19 (FGF19), or its rodent homologue FGF15, when injected centrally, improves glucose tolerance in obese rats [27]. Similarly, perfusion of the incretin glucagon-like peptide 1 (GLP-1) in the Arc causes a decrease in HGP [28]. Recent studies have also reported involvement of the hypothalamic neuropeptide orexin in the brain regulation of glucose metabolism. Indeed, central administration of orexin A during the night-time awake period first elevates blood glucose levels and subsequently lowers daytime glycaemia in normal and diabetic db/db mice by bidirectional regulation of HGP relayed by the autonomic nervous system [29, 30]. Collectively, these observations support the hypothesis that the control of glucose homeostasis results from complex and highly coordinated interactions between the brain and the pancreatic islets.
26RFa is interesting in that it is produced not only at the periphery by the gut and the pancreatic islets [23,24,25], but also by a discrete neuronal population located in the hypothalamus [12, 16, 18, 19]. This observation raises the possibility that 26RFa-producing neurons belong to the glucoregulatory system of the hypothalamus and are responsible for the central effects of the peptide on glucose homeostasis. To test this hypothesis, we investigated whether peripheral factors that are well known to control the regulation of glucose metabolism by the hypothalamus can modulate the expression and secretion of 26RFa by 26Rfa-expressing neurons in hypothalamic explants. Our experiments revealed that neither glucose nor leptin substantially impairs 26RFa secretion. Conversely, we found that insulin drastically stimulates the secretion of 26RFa by the hypothalamus. This finding strongly suggests that the 26RFa neuronal circuitry belongs to the glucoregulatory system of the hypothalamus and may relay, at least in part, the central effects of insulin on the regulation of glucose metabolism. Consistent with this hypothesis, we show that central 26RFa displays a very similar antihyperglycaemic effect to that of insulin in terms of amplitude and kinetics, and that the central antihyperglycaemic effect of insulin is abolished in the presence of a 26RFa receptor antagonist and in mice deficient for 26RFa. In addition, we show that the main population of 26Rfa-expressing neurons is localised in the LHA, with two subpopulations present in the VMH and the basal part of the tuber cinereum area, whereas 26Rfa-expressing neurons are absent in the Arc. We also observed that a number of these 26Rfa-expressing neurons also express Insr and that central administration of insulin induces the expression of 26RFa in neuronal populations of the hypothalamus. Altogether, these complementary observations strongly suggest that 26Rfa-expressing neurons are a target for insulin and mediate the regulatory effect of the hormone on glucose homeostasis within the hypothalamus. Interestingly, it was previously shown that injection of insulin into the Arc lowers blood glucose levels, increases insulin sensitivity in the liver [5, 6] and decreases HGP [7]. The AgRP neurons of the Arc were identified as one of the neuronal populations targeted by insulin, as specific deletion of the insulin receptor in AgRP neurons partially blunts the ability of insulin to inhibit HGP [11]. By contrast, ablation of the insulin receptor in the POMC neurons of the Arc or specific activation of these neurons has no effect on glycaemia nor on HGP [11, 31]. Here, we provide evidence that insulin targets a new discrete population of hypothalamic neurons that are located not in the Arc but in the LHA: the 26Rfa-expressing neurons.
It is well documented that the central antihyperglycaemic action of insulin is due, in part, to an inhibitory effect of the hormone on HGP [7]. We therefore examined whether 26RFa-expressing neurons mediate the central insulin effects on HGP. We found that 26RFa injected centrally has no effect on HGP, in contrast to insulin, and that the effect of insulin on HGP is not impaired by a blockade of the 26RFa receptor, nor in 26RFa-deficient mice. However, it has been reported that the central antihyperglycaemic effect of insulin is also due to activation of its own production by the pancreatic islets [32]. Our results support this as we show that insulin injected centrally significantly increases plasma insulin levels and that the central antihyperglycaemic effect of insulin is totally abolished in streptozotocin-treated mice that are unable to produce and secrete insulin. In addition, we show that GPR103 blockade or the absence of 26RFa (in 26Rfa–/– mice) abolishes insulin-induced insulin production. These observations indicate that the hypothalamic 26Rfa-expressing neurons are not involved in the central inhibitory effect of insulin on HGP but mediate the central effects of insulin on its own peripheral production. The present findings, together with previous findings, support the original hypothesis that insulin is able to target distinct neuronal populations in the hypothalamus, that is, the AgRP neurons in the Arc that mediate the inhibitory effect of the hormone on HGP, and the 26RFa neurons in the LHA that mediate the stimulatory action of insulin on its own peripheral production. We propose that these two synergical actions may be responsible for the central antihyperglycaemic effect of insulin.
In conclusion, in the present study we identify a novel hypothalamic neuronal circuit that regulates glucose homeostasis, the 26RFa/GPR103 neuropeptidergic system, and provide evidence that this discrete neuronal population is a key relay for the central regulation of glucose metabolism by insulin.
Data availability
All data generated or analysed during this study are included in the published article (and its supplementary information files).
Abbreviations
- 26RFa:
-
Pyroglutamilated RFamide peptide (QRFP)
- ACD:
-
Advanced Cell Diagnostics
- AgRP:
-
Agouti-related peptide
- AGSIS:
-
Acute glucose-stimulated insulin secretion
- Arc:
-
Arcuate nucleus
- Ct:
-
Comparative threshold
- HGP:
-
Hepatic glucose production
- i.c.v.:
-
Intracerebroventricular
- LHA:
-
Lateral hypothalamic area
- POMC:
-
Proopiomelanocortin
- PTT:
-
Pyruvate tolerance test
- qRT-PCR:
-
Quantitative reverse transcription PCR
- TFA:
-
Trifluoroacetic acid
- VMH:
-
Ventromedial hypothalamic nucleus
References
Bernard C (1854) Leçons de physiologie expérimentales appliquées à la médecine. JB Baillière, Paris
Schwartz MW, Seeley RJ, Tschöp MH et al (2013) Cooperation between brain and islet in glucose homeostasis and diabetes. Nature 503:59–66. https://doi.org/10.1038/nature12709
Carey M, Kehlenbrink S, Hawkins M (2013) Evidence for central regulation of glucose metabolism. J Biol Chem 288:34981–34988. https://doi.org/10.1074/jbc.R113.506782
Fujikawa T, Berglund ED, Patel VR et al (2013) Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab 18(3):431–444. https://doi.org/10.1016/j.cmet.2013.08.004
Obici S, Zhang BB, Karkanias G et al (2002) Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382. https://doi.org/10.1038/nm1202-798
Lam TK, Gutierrez-Juarez R, Pocai A et al (2005) Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 309:943–947. https://doi.org/10.1126/science.1112085
Inoue H (2016) Central insulin-mediated regulation of HGP. Endocr J 63:1–7. https://doi.org/10.1507/endocrj.EJ15-0540
Morton GJ, Gelling RW, Niswender KD et al (2005) Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab 2:411–420. https://doi.org/10.1016/j.cmet.2005.10.009
Jordan SD, Könner AC, Brüning JC (2010) Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cell Mol Life Sci 67:3255–3273. https://doi.org/10.1007/s00018-010-0414-7
Hill JW, Elias CF, Fukuda M et al (2010) Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 11:286–297. https://doi.org/10.1016/j.cmet.2010.03.002
Könner AC, Janoschek R, Plum L et al (2007) Insulin action in AgRP-expressing neurons is required for suppression of HGP. Cell Metab 5:438–449. https://doi.org/10.1016/j.cmet.2007.05.004
Chartrel N, Dujardin C, Anouar Y et al (2003) Identification of 26RFa, a hypothalamic neuropeptide of the RFamide peptide family with orexigenic activity. Proc Natl Acad Sci U S A 100:15247–15252. https://doi.org/10.1073/pnas.2434676100
Fukusumi S, Yoshida H, Fujii R et al (2003) A new peptidic ligand and its receptor regulating adrenal function in rats. J Biol Chem 278:46387–46395. https://doi.org/10.1074/jbc.M305270200
Jiang Y, Luo L, Gustafson EL et al (2003) Identification and characterization of a novel RF-amide peptide ligand for orphan G-protein-coupled receptor SP9155. J Biol Chem 278:27652–27657. https://doi.org/10.1074/jbc.M302945200
Chartrel N, Alonzeau J, Alexandre D et al (2011) The RFamide neuropeptide 26RFa and its role in the control of neuroendocrine functions. Front Neuroendocrinol 32:387–397. https://doi.org/10.1016/j.yfrne.2011.04.001
Takayasu S, Sakurai T, Iwasaki S et al (2006) A neuropeptide ligand of the G protein-coupled receptor GPR103 regulates feeding, behavioral arousal, and blood pressure in mice. Proc Natl Acad Sci U S A 103:7438–7443. https://doi.org/10.1073/pnas.0602371103
Leprince J, Bagnol D, Bureau R et al (2017) The Arg-Phe-amide peptide 26RFa/glutamine RF-amide peptide and its receptor: IUPHAR Review 24. Br J Pharmacol 174(20):3573–3607. https://doi.org/10.1111/bph.13907
Bruzzone F, Lectez B, Tollemer H et al (2006) Anatomical distribution and biochemical characterization of the novel RFamide peptide 26RFa in the human hypothalamus and spinal cord. J Neurochem 99:616–627. https://doi.org/10.1111/j.1471-4159.2006.04090.x
Bruzzone F, Lectez B, Alexandre D et al (2007) Distribution of 26RFa binding sites and GPR103 mRNA in the central nervous system of the rat. J Comp Neurol 503:573–591. https://doi.org/10.1002/cne.21400
Moriya R, Sano H, Umeda T et al (2006) RFamide peptide QRFP43 causes obesity with hyperphagia and reduced thermogenesis in mice. Endocrinology 147:2916–2922. https://doi.org/10.1210/en.2005-1580
Lectez B, Jeandel L, El-Yamani FZ et al (2009) The orexigenic activity of the hypothalamic neuropeptide 26RFa is mediated by the neuropeptide Y and proopiomelanocortin neurons of the arcuate nucleus. Endocrinology 150:2342–2350. https://doi.org/10.1210/en.2008-1432
Granata R, Settanni F, Trovato L et al (2014) RFamide peptides 43RFa and 26RFa both promote survival of pancreatic β-cells and human pancreatic islets but exert opposite effects on insulin secretion. Diabetes 63:2380–2393. https://doi.org/10.2337/db13-1522
Prévost G, Jeandel L, Arabo A et al (2015) Hypothalamic Neuropeptide 26RFa Acts as an Incretin to Regulate Glucose Homeostasis. Diabetes 64:2805–2816. https://doi.org/10.2337/db14-1864
Prévost G, Picot M, Le Solliec MA et al (2019) The neuropeptide 26RFa in the human gut and pancreas: potential involvement in glucose homeostasis. Endocr Connect 8:941–951. https://doi.org/10.1530/EC-19-0247
Prévost G, Arabo A, Le Solliec MA et al (2019) Neuropeptide 26RFa (QRFP) is a key regulator of glucose homeostasis and its activity is markedly altered in obese/hyperglycemic mice. Am J Physiol Endocrinol Metab 317(1):E147–E157. https://doi.org/10.1152/ajpendo.00540.2018
El Mehdi M, Takhlidjt S, Khiar F et al (2020) Glucose homeostasis is impaired in mice deficient for the neuropeptide 26RFa (QRFP). BMJ Open Diabetes Res Care 8:e000942. https://doi.org/10.1136/bmjdrc-2019-000942
Ryan KK, Kohli R, Gutierrez-Aguilar R et al (2013) Fibroblast growth factor-19 action in the brain reduces food intake and body weight and improves glucose tolerance in male rats. Endocrinology 154:9–15. https://doi.org/10.1210/en.2012-1891
Prigeon RL, Quddusi S, Paty B et al (2003) Suppression of glucose production by GLP-1 independent of islet hormones: a novel extrapancreatic effect. Am J Physiol Endocrinol Metab 285:E701–E707. https://doi.org/10.1152/ajpendo.00024.2003
Tsuneki H, Tokai E, Nakamura Y et al (2015) Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes 64:459–470. https://doi.org/10.2337/db14-0695
Rani M, Kumar R, Krishan P (2018) Role of orexins in the central and peripheral regulation of glucose homeostasis: Evidences & mechanisms. Neuropeptides 68:1–6. https://doi.org/10.1016/j.npep.2018.02.002
Steculorum SM, Ruud J, Karakasilioti I et al (2016) AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165:125–138. https://doi.org/10.1016/j.cell.2016.02.044
Scherer T, Sakamoto K, Buettner C (2021) Brain insulin signaling in metabolic homeostasis and disease. Nat Rev Endocrinol 17:468–483. https://doi.org/10.1038/s41574-021-00498-x
Data availability
All data generated or analysed during this study are included in the published article (and its supplementary information files).
Funding
This work was supported by Inserm (U1239), the University of Rouen, the Institute for Research and Innovation in Biomedicine (IRIB), the Agence Nationale pour la Recherche (DIABNET), the Fondation pour la Recherche Médicale (grant no. DEA 20140629966), the Société Francophone du Diabète (grant no. R16038EE) and the Plateforme de Recherche en Imagerie Cellulaire de Normandie (PRIMACEN). The present study was also co-funded by the European Union and the Regional Council of Normandy. The European Union is involved with the Regional Council of Normandy through the European Regional Development Fund (ERDF).
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Funding
This work was supported by Inserm (U1239), the University of Rouen, the Institute for Research and Innovation in Biomedicine (IRIB), the Agence Nationale pour la Recherche (DIABNET), the Fondation pour la Recherche Médicale (grant no. DEA 20140629966), the Société Francophone du Diabète (grant no. R16038EE) and the Plateforme de Recherche en Imagerie Cellulaire de Normandie (PRIMACEN). The present study was also co-funded by the European Union and the Regional Council of Normandy. The European Union is involved with the Regional Council of Normandy through the European Regional Development Fund (ERDF).
Author information
Authors and Affiliations
Contributions
MEM, MP and NC contributed substantially to the study conception and design and analysis and interpretation of data and wrote the manuscript. ST, JM, AA, MP, M-ALS and MEM performed and analysed the in vivo experiments in mice. ST, CD, MD and MP performed and analysed the RNAscope experiments. MD and MEM performed and analysed the PCR experiments. AB and EN performed and analysed the insulin assays, and JL and BL produced synthetic 26RFa. GP, JL and YA contributed to the study design and critically revised the manuscript for important intellectual content. All authors contributed substantially to the drafting of the manuscript and approved the final version of the paper. NC is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM
(PDF 218 kb)
Rights and permissions
About this article
Cite this article
El Mehdi, M., Takhlidjt, S., Devère, M. et al. The 26RFa (QRFP)/GPR103 neuropeptidergic system in mice relays insulin signalling into the brain to regulate glucose homeostasis. Diabetologia 65, 1198–1211 (2022). https://doi.org/10.1007/s00125-022-05706-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-022-05706-5