Abstract
Key message
Plastidial α-glucan phosphorylase is a key factor that cooperates with plastidial disproportionating enzyme to control short maltooligosaccharide mobilization during the initiation process of starch molecule synthesis in developing rice endosperm.
Abstract
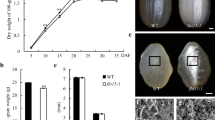
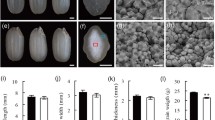
Storage starch synthesis is essential for grain filling. However, little is known about how cereal endosperm controls starch synthesis initiation. One of core events for starch synthesis initiation is short maltooligosaccharide (MOS) mobilization consisting of long MOS primer production and excess MOS breakdown. By mutant analyses and biochemical investigations, we present here functional identifications of plastidial α-glucan phosphorylase (Pho1) and disproportionating enzyme (DPE1) during starch synthesis initiation in rice (Oryza sativa) endosperm. Pho1 deficiency impaired MOS mobilization, triggering short MOS accumulation and starch synthesis reduction during early seed development. The mutant seeds differed significantly in MOS level and starch content at 15 days after flowering and exhibited diverse endosperm phenotypes during mid-late seed development: ranging from pseudonormal to shrunken (Shr), severely or excessively Shr. The level of DPE1 was almost normal in the PN seeds but significantly reduced in the Shr seeds. Overexpression of DPE1 in pho1 resulted in plump seeds only. DPE1 deficiency had no obvious effects on MOS mobilization. Knockout of DPE1 in pho1 completely blocked MOS mobilization, resulting in severely and excessively Shr seeds only. These findings show that Pho1 cooperates with DPE1 to control short MOS mobilization during starch synthesis initiation in rice endosperm.








Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper and within its supplementary materials published online.
Abbreviations
- DAF:
-
Days after flowering
- DP:
-
Degree of polymerization
- MOS:
-
Maltooligosaccharide
- PN:
-
Pseudonormal
- Shr:
-
Shrunken
- WC:
-
White-core
- WT:
-
Wild type
References
Abt MR, Pfister B, Sharma M et al (2020) STARCH SYNTHASE 5, a noncanonical starch synthase-like protein, promotes starch granule initiation in Arabidopsis. Plant Cell 32:2543–2565
Ball SG, Morell MK (2003) From bacterial glycogen to starch: understanding the biogenesis of the plant starch granule. Annu Rev Plant Biol 54:207–233
Brust H, Orzechowski S, Fettke J, Steup M (2013) Starch synthesizing reactions and paths: in vitro and in vivo studies. J Appl Glycosci 60:2–20
Cakir B, Shiraishi S, Tuncel A et al (2016) Analysis of the rice ADP-glucose transporter (OsBT1) indicates the presence of regulatory processes in the amyloplast stroma that control ADP-glucose flux into starch. Plant Physiol 170:1271–1283
Cao H, James MG, Myers AM (2000) Purification and characterization of soluble starch synthases from maize endosperm. Arch Biochem and Biophys 373:135–146
Critchley JH, Zeeman SC, Takaha T, Smith AM, Smith SM (2001) A critical role for disproportionating enzyme in starch breakdown is revealed by a knock-out mutation in Arabidopsis. Plant J 26:89–100
Crofts N, Nakamura Y, Fujita N (2017) Critical and speculative review of the roles of multi-protein complexes in starch biosynthesis in cereals. Plant Sci 262:1–8
Crumpton-Taylor M, Pike M, Lu K, Hylton CM, Feil R, Eicke S, Lunn J, Zeeman S, Simith A (2013) Starch synthase 4 is essential for coordination of starch granule formation with chloroplast division during Arabidopsis leaf expansion. New Phytol 200:1064–1075
D’Hulst C, Mérida Á (2010) The priming of storage glucan synthesis from bacteria to plants: current knowledge and new developments. New Phytol 188:13–21
Dong X, Li J, Li S, Zhang J, Hua Z-C (2008) A novel genotyping strategy based on allele-specific inverse PCR for rapid and reliable identification of conditional FADD knockout mice. Mol Biotechnol 38:129–135
Dong X, Zhang D, Liu J, Liu QQ, Liu HL, Tian L, Jiang L, Qu LQ (2015) Plastidial disproportionating enzyme participates in starch synthesis in rice endosperm by transferring maltooligosyl groups from amylose and amylopectin to amylopectin. Plant Physiol 169:2496–2512
Dumez S, Wattebled F, Dauvillee D, Delvalle D, Planchot V, Ball SG, D’Hulst C (2006) Mutants of Arabidopsis lacking starch branching enzyme II substitute plastidial starch synthesis by cytoplasmic maltose accumulation. Plant Cell 18:2694–2709
Fujita N, Kubo A, Francisco PB, Nakakita M, Harada K, Minaka N, Nakamura Y (1999) Purification, characterization, and cDNA structure of isoamylase from developing endosperm of rice. Planta 208:283–293
Fujita N, Yoshida M, Asakura N, Ohdan T, Miyao A, Hirochika H, Nakamura Y (2006) Function and characterization of starch synthase I using mutants in rice. Plant Physiol 140:1070–1084
Fujita N, Yoshida M, Kondo T et al (2007) Characterization of SSIIIa deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol 144:2009–2023
Hayashi M, Suzuki R, Colleoni C, Ball SG, Fujita N, Suzuki E (2017) Bound substrate in the structure of Cyanobacterial branching enzyme supports a new mechanistic model. J Biol Chem 292:5465–5475
Hizukuri S (1986) Polymodal distribution of the chain lengths of amylopectins and its significance. Carbohyd Res 147:342–347
Hwang SK, Nishi A, Satoh H, Okita TW (2010) Rice endosperm-specific plastidial α-glucan phosphorylase is important for synthesis of short-chain malto-oligosaccharides. Arch Biochem Biophys 495:82–92
Hwang SK, Koper K, Satoh H, Okita TW (2016a) Rice endosperm starch phosphorylase (Pho1) assembles with disproportionating enzyme (Dpe1) to form a protein complex that enhances synthesis of malto-oligosaccharides. J Biol Chem 291:19994–20007
Hwang SK, Singh S, Cakir B, Satoh H, Okita TW (2016b) The plastidial starch phosphorylase from rice endosperm: catalytic properties at low temperature. Planta 243:999–1009
James MG, Denyer K, Myers AM (2003) Starch synthesis in the cereal endosperm. Curr Opin Plant Biol 6:215–222
Jeon JS, Ryoo N, Hahn TR, Walia H, Nakamura Y (2010) Starch biosynthesis in cereal endosperm. Plant Physiol Biochem 48:383–392
Kawagoe Y, Kubo A, Satoh H, Takaiwa F, Nakamura Y (2005) Roles of isoamylase and ADP glucose pyrophosphorylase in starch granule synthesis in rice endosperm. Plant J 42:164–174
Kitamura S, Yunokawa H, Mitsuie S, Kuge T (1982) Study on polysaccharides by the fluorescence method. II. Micro-Brownian motion and conformational change of amylase in aqueous solution. Polym J 14:93–99
Ma Z, Chen G, Wu C (2010) Development of a Tos17 insertional mutant library in the rice variety Zhonghua 11. Mol Plant Breed 8:1–10
Ma X, Zhang Q, Zhu Q et al (2015) A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant 8:1274–1284
Malinova I, Alseekh S, Feil R et al (2017) Starch synthase 4 and plastidal phosphorylase differentially affect starch granule number and morphology. Plant Physiol 174:73–85
Nakamura Y (2002) Towards a better understanding of the metabolic system for amylopectin biosynthesis in plants: rice endosperm as a model tissue. Plant Cell Physiol 43:718–725
Nakamura Y, Utsumi Y, Sawada T, Aihara S, Utsumi C, Yoshida M, Kitamura S (2010) Reaction characterization of starch branching enzymes from rice endosperm. Plant Cell Physiol 51:776–794
Nakamura Y, Ono M, Utsumi Y, Steup M (2012) Functional interaction between plastidial starch phosphorylase and starch branching enzymes from rice during the synthesis of branched maltodextrins. Plant Cell Physiol 53:869–878
Nakamura Y, Aihara S, Crofts N, Swada T, Fujita N (2014) In vitro studies of enzymatic properties of starch synthases and interactions between starch synthase I and starch branching enzymes from rice. Plant Sci 224:1–8
Nakamura Y, Ono M, Sawada T, Crofts N, Fujita N, Steup M (2017) Characterization of the functional interactions of plastidial starch phosphorylase and starch branching enzymes from rice endosperm during reserve starch biosynthesis. Plant Sci 264:83–95
Ohdan T, Francisco PB Jr, Sawada T, Hirose T, Terao T, Satoh H, Nakamura Y (2005) Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J Exp Bot 56:3229–3244
Pico J, Martínez MM, Martín MT, Gómez M (2015) Quantification of sugars in wheat flours with an HPAEC-PAD method. Food Chem 173:674–681
Preiss J, Sivak M (1996) Starch synthesis in sinks and sources. In: Zamski E, Schaffer AA (eds) Photoassimilate distribution in plants and crops: source-sink relationships. Marcel Dekker, New York, pp 139–168
Qu LQ, Tada Y, Takaiwa F (2003) In situ Western hybridization: a new, highly sensitive technique to detect foreign and endogenous protein distribution in rice seeds. Plant Cell Rep 22:282–285
Qu LQ, Xing YP, Liu WX, Xu XP, Song YR (2008) Expression pattern and activity of six glutelin gene promoters in transgenic rice. J Exp Bot 59:2417–2424
Ragel P, Streb S, Feil R, Sahrawy M, Annunziata MG, Lunn JE, Zeeman S, Mérida Á (2013) Loss of starch granule initiation has a deleterious effect on the growth of Arabidopsis plants due to an accumulation of ADP-glucose. Plant Physiol 163:75–85
Roldán L, Wattebled F, Lucas MM, Delvallé D, Planchot V, Jiménez S, Pérez R, Ball S, D’Hulst C, Mérida Á (2007) The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J 49:492–504
Satoh H, Nishi A, Yamashita K, Takemoto Y, Tanaka Y, Hosaka Y, Sakurai A, Fujita N, Nakamura Y (2003) Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiol 133:1111–1121
Satoh H, Shibahara K, Tokunaga T et al (2008) Mutation of the plastidial alpha-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell 20:1833–1849
Sawada T, Nakamura Y, Ohdan T et al (2014) Diversity of reaction characteristics of glucan branching enzymes and the fine structure of α-glucan from various sources. Arch Biochem Biophys 562:9–21
Seung D, Lu KJ, Stettler M, Streb S, Zeeman SC (2016) Degradation of glucan primers in the absence of starch synthase 4 disrupts starch granule initiation in Arabidopsis. J Biol Chem 291:20718–20728
Seung D, Boudet J, Monroe J et al (2017) Homologs of PROTEIN TARGETING TO STARCH control starch granule initiation in Arabidopsis leaves. Plant Cell 29:1657–1677
Seung D, Schreier TB, Léo B, Simona E, Zeeman SC (2018) Two plastidial oiled-coil proteins are essential for normal starch granule initiation in Arabidopsis. Plant Cell 30:1523–1542
Sikka VK, Choi S, Kavakli IH, Sakulsingharoj C, Gupta S, Ito H, Okita TW (2001) Subcellular compartmentation and allosteric regulation of the rice endosperm ADP glucose pyrophosphorylase. Plant Sci 161:461–468
Stettler M, Eicke S, Mettler T, Messerli G, Hörtensteiner Zeeman SC (2009) Blocking the metabolism of starch breakdown products in Arabidopsis leaves triggers chloroplast degradation. Mol Plant 2:1233–1246
Szydlowski N, Ragel P, Raynaud S et al (2009) Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthases. Plant Cell 21:2443–2457
Tetlow IJ (2006) Understanding storage starch biosynthesis in plants: a means to quality improvement. Can J Bot 84:1167–1187
Tetlow IJ, Wait R, Lu Z, Akkasaeng R, Bowsher CG, Esposito S, Kosar-Hashemi B, Morell MK, Emes MJ (2004) Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell 16:694–708
Thompson DB (2000) On the non-random nature of amylopectin branching. Carbohyd Polym 43:223–239
Tuncel A, Kawaguchi J, Ihara Y et al (2014) The rice endosperm ADP glucose pyrophosphorylase large subunit is essential for optimal catalysis and allosteric regulation of the heterotetrameric enzyme. Plant Cell Physiol 55:1169–1183
Turner DH, Turner JF (1960) The hydrolysis of Glc monophosphates by a phosphatase preparation from pea seeds. Biochem J 74:486–491
Utsumi Y, Utsumi C, Sawada T, Fujita N, Nakamura Y (2011) Functional diversity of isoamylase oligomers: the ISA1 homo-oligomer is essential for amylopectin biosynthesis in rice endosperm. Plant Physiol 156:61–77
Wu AC, Li EP, Gilbert RG (2014) Exploring extraction/dissolution procedures for analysis of starch chain-length distributions. Carbohydr Polym 114:36–42
Yamanouchi H, Nakamura Y (1992) Organ specificity of isoforms of starch branching enzyme (Q-enzyme) in rice. Plant Cell Physiol 33:985–991
Yang T, Tan X, Huang S, Pan X et al (2022) Effects of experimental warming on physicochemical properties of indica rice starch in a double rice cropping system. Food Chem 310:125981
Acknowledgements
We thank Mr. Tong Sun (Institute of Microbiology, Chinese Academy of Sciences) for his excellent technical assistance on HPAEC-PAD. We thank Ms. Li Wang and Ms. Jindan Zhang (Institute of Botany, Chinese Academy of Sciences) for their help with protein purification and starch fine structure.
Funding
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDA24010401) to L.Q.Q., the Natural Science Foundation of China (No. 31771697, 31070226) to X.B.D., and National Program of Transgenic Variety Development of China (2016ZX08001-006) to L.Q.Q.
Author information
Authors and Affiliations
Contributions
X.D. and L.Q.Q. designed the research and wrote the article. X.D. performed most of the experiments. L.C. performed some experiments. X.D. analyzed the data. H.Y., L.T., F.D. and Y.C. provided technical assistance to X.D. All authors reviewed the results and approved to submit the manuscript to your journal.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interests.
Additional information
Communicated by Ian D. Godwin.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, X., Chen, L., Yang, H. et al. Pho1 cooperates with DPE1 to control short maltooligosaccharide mobilization during starch synthesis initiation in rice endosperm. Theor Appl Genet 136, 47 (2023). https://doi.org/10.1007/s00122-023-04250-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04250-z