Abstract
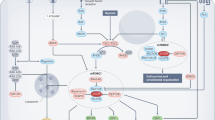
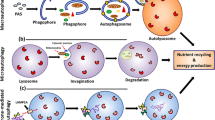
Patients treated with the mammalian or mechanistic target of rapamycin (mTOR) inhibitor everolimus in order to slow progression of autosomal-dominant polycystic kidney disease (ADPKD) showed a significant reduction of body weight. Although the detailed mechanism of how mTOR inhibition interferes with body weight regulation is rather unclear, present data suggest that this effect is mediated by both central and peripheral mechanisms. These findings in ADPKD patients are in contrast to well-documented effects of hypothalamic mTOR on regulation of energy homeostasis and eating behavior in rodents. In a number of rodent models, the mTOR inhibitor rapamycin induces increased food intake, which is accompanied by increased body weight. However, animal data are inconsistent. This review highlights some of the regulatory signals and key mechanisms that are important for balancing energy intake and energy expenditure with a special focus on adipose tissue-derived adipokines and their interaction with mTOR regarding local regulation of tissue perfusion and metabolism and overall systemic energy homeostasis. Specifically, clinical aspects of an impaired mTOR signaling pathway regarding the development of obesity and type-2 diabetes mellitus will be discussed.



Similar content being viewed by others
Notes
Mammalian or mechanistic target of rapamycin
References
Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A et al (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A 103:5466–5471
Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D (2003) Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol 5:578–581
Pan D, Dong J, Zhang Y, Gao X (2004) Tuberous sclerosis complex: from Drosophila to human disease. Trends Cell Biol 14:78–85
Li Y, Corradetti MN, Inoki K, Guan KL (2004) TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci 29:32–38
Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N et al (2010) Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363:830–840
Rovira J, Marcelo Arellano E, Burke JT, Brault Y, Moya-Rull D, Banon-Maneus E, Ramirez-Bajo MJ, Gutierrez-Dalmau A, Revuelta I, Quintana LF et al (2008) Effect of mTOR inhibitor on body weight: from an experimental rat model to human transplant patients. Transpl Int Off J Eur Soc Organ Transplant 21:992–998
Dabora SL, Franz DN, Ashwal S, Sagalowsky A, DiMario FJ Jr, Miles D, Cutler D, Krueger D, Uppot RN, Rabenou R et al (2011) Multicenter phase 2 trial of sirolimus for tuberous sclerosis: kidney angiomyolipomas and other tumors regress and VEGF-D levels decrease. PLoS One 6:e23379
Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, Maeder C, Fournier M, Montet X, Rohner-Jeanrenaud F et al (2012) Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol 165:2325–2340
Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N et al (2008) mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes 57:945–957
Kramer S, Wang-Rosenke Y, Scholl V, Binder E, Loof T, Khadzhynov D, Kawachi H, Shimizu F, Diekmann F, Budde K et al (2008) Low-dose mTOR inhibition by rapamycin attenuates progression in anti-thy1-induced chronic glomerulosclerosis of the rat. Am J Physiol Ren Physiol 294:F440–F449
Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP (2006) Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 21:598–604
Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV (2010) Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol 176:2092–2097
Korfhagen TR, Le Cras TD, Davidson CR, Schmidt SM, Ikegami M, Whitsett JA, Hardie WD (2009) Rapamycin prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 41:562–572
Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, Mao FC (2009) Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci 109:496–503
Blouet C, Ono H, Schwartz GJ (2008) Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metabol 8:459–467
Cota D, Proulx K, Smith KAB, Kozma SC, Thomas G, Woods SC, Seeley RJ (2006) Hypothalamic mTOR signaling regulates food intake. Science 312:927–930
Kahn BB, Myers MG (2006) mTOR tells the brain that the body is hungry. Nat Med 12:615–617
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW (2006) Central nervous system control of food intake and body weight. Nature 443:289–295
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG et al (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364:514–523
Seeliger H, Guba M, Kleespies A, Jauch KW, Bruns CJ (2007) Role of mTOR in solid tumor systems: a therapeutical target against primary tumor growth, metastases, and angiogenesis. Cancer Metastasis Rev 26:611–621
Chang SH, McDonald SP (2008) Post-kidney transplant weight change as marker of poor survival outcomes. Transplantation 85:1443–1448
Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS et al (2012) Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335:1638–1643
Figlin RA, Kaufmann I, Brechbiel J (2013) Targeting PI3K and mTORC2 in metastatic renal cell carcinoma: New strategies for overcoming resistance to VEGFR and mTORC1 inhibitors. Int J Cancer. doi:10.1002/ijc.28023
Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J (2008) Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA 105:17414–17419
Dennis MD, Kimball SR, Jefferson LS (2013) Mechanistic target of rapamycin complex 1 (mTORC1)-mediated phosphorylation is governed by competition between substrates for interaction with raptor. J Biol Chem 288:10–19
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol CB 14:1296–1302
Cota D, Marsicano G, Tschöp M, Grübler Y, Flachskamm C, Schubert M, Auer D, Yassouridis A, Thöne-Reineke C, Ortmann S et al (2003) The endogenous cannabinoid system affects energy balance via central orexigenic drive and peripheral lipogenesis. J Clin Invest 112:423–431
Ropelle ER, Pauli JR, Fernandes MFA, Rocco SA, Marin RM, Morari J, Souza KK, Dias MM, Gomes-Marcondes MC, Gontijo JAR et al (2008) A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes 57:594–605
Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G (2001) Mammalian TOR: a homeostatic ATP sensor. Science 294:1102–1105
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432
Schwartz MW, Erickson JC, Baskin DG, Palmiter RD (1998) Effect of fasting and leptin deficiency on hypothalamic neuropeptide Y gene transcription in vivo revealed by expression of a lacZ reporter gene. Endocrinology 139:2629–2635
Schwartz MW, Porte D Jr (2005) Diabetes, obesity, and the brain. Science 307:375–379
Kadowaki T, Yamauchi T, Kubota N (2008) The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 582:74–80
Kopin AS, Mathes WF, McBride EW, Nguyen M, Al-Haider W, Schmitz F, Bonner-Weir S, Kanarek R, Beinborn M (1999) The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J Clin Invest 103:383–391
Little TJ, Horowitz M, Feinle-Bisset C (2005) Role of cholecystokinin in appetite control and body weight regulation. Obes Rev Off J Int Assoc Study Obes 6:297–306
Weyer C, Maggs DG, Young AA, Kolterman OG (2001) Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Curr Pharm Des 7:1353–1373
Barth SW, Riediger T, Lutz TA, Rechkemmer G (2004) Peripheral amylin activates circumventricular organs expressing calcitonin receptor a/b subtypes and receptor-activity modifying proteins in the rat. Brain Res 997:97–102
Roth JD, Erickson MR, Chen S, Parkes DG (2012) GLP-1R and amylin agonism in metabolic disease: complementary mechanisms and future opportunities. Br J Pharmacol 166:121–136
Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S (2001) A role for ghrelin in the central regulation of feeding. Nature 409:194–198
Kharitonenkov A, Larsen P (2011) FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab TEM 22:81–86
Lim CT, Kola B, Korbonits M (2010) AMPK as a mediator of hormonal signalling. J Mol Endocrinol 44:87–97
Andersson U, Filipsson K, Abbott CR, Woods A, Smith K, Bloom SR, Carling D, Small CJ (2004) AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 279:12005–12008
Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB et al (2005) Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 280:25196–25201
Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T et al (2007) Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6:55–68
Wen JP, Liu CE, Hu YT, Chen G, Lin LX (2010) Globular adiponectin regulates energy homeostasis through AMP-activated protein kinase-acetyl-CoA carboxylase (AMPK/ACC) pathway in the hypothalamus. Mol Cell Biochem 344:109–115
Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ et al (2004) AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428:569–574
Scerif M, Goldstone AP, Korbonits M (2011) Ghrelin in obesity and endocrine diseases. Mol Cell Endocrinol 340:15–25
Boschmann M, Mannaa M, Pfennigwerth P, Gaedeke J, Budde K, Gollasch M (2011) Everolimus und Energiemetabolismus bei Patienten mit autosomal-dominant vererbbarer Zystennierenerkrankung. 3 Jahrestagung der Deutschen Gesellschaft für Nephrologie, Berlin A44-11/1424
Fliedner S, Schulz C, Lehnert H (2006) Brain uptake of intranasally applied radioiodinated leptin in Wistar rats. Endocrinology 147:2088–2094
Banks WA (2001) Leptin transport across the blood–brain barrier: implications for the cause and treatment of obesity. Curr Pharm Des 7:125–133
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S et al (1995) Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161
Sweeney G (2002) Leptin signalling. Cell Signal 14:655–663
Wauman J, Tavernier J (2011) Leptin receptor signaling: pathways to leptin resistance. Front Biosci J Virtual Lib 17:2771–2793
Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A (2011) Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab 301:E567–E584
Marshall S (2006) Role of insulin, adipocyte hormones, and nutrient-sensing pathways in regulating fuel metabolism and energy homeostasis: a nutritional perspective of diabetes, obesity, and cancer. Sci STKE Sig Transduct Knowl Environ 2006:re7. doi:10.1126/stke.3462006re7
Cota D, Matter EK, Woods SC, Seeley RJ (2008) The role of hypothalamic mammalian target of rapamycin complex 1 signaling in diet-induced obesity. J Neurosci 28:7202–7208
Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F (1995) Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543
Cho HJ, Park J, Lee HW, Lee YS, Kim JB (2004) Regulation of adipocyte differentiation and insulin action with rapamycin. Biochem Biophys Res Commun 321:942–948
Bradley RL, Cheatham B (1999) Regulation of ob gene expression and leptin secretion by insulin and dexamethasone in rat adipocytes. Diabetes 48:272–278
Hinault C, Mothe-Satney I, Gautier N, Lawrence JC Jr, Van Obberghen E (2004) Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB Off Publ Fed Am Soc Exp Biol 18:1894–1896
Burguera B, Couce ME, Curran GL, Jensen MD, Lloyd RV, Cleary MP, Poduslo JF (2000) Obesity is associated with a decreased leptin transport across the blood–brain barrier in rats. Diabetes 49:1219–1223
Vila R, Adan C, Rafecas I, Fernandez-Lopez JA, Remesar X, Alemany M (1998) Plasma leptin turnover rates in lean and obese Zucker rats. Endocrinology 139:4466–4469
Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749
Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M (2007) Adiponectin is a novel humoral vasodilator. Cardiovasc Res 75:719–727
Coope A, Milanski M, Araujo EP, Tambascia M, Saad MJ, Geloneze B, Velloso LA (2008) AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett 582:1471–1476
Takeuchi T, Adachi Y, Ohtsuki Y, Furihata M (2007) Adiponectin receptors, with special focus on the role of the third receptor, T-cadherin, in vascular disease. Med Mol Morphol 40:115–120
Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116:1784–1792
Rabe K, Lehrke M, Parhofer KG, Broedl UC (2008) Adipokines and insulin resistance. Mol Med 14:741–751
Suzuki M, Uehara Y, Motomura-Matsuzaka K, Oki J, Koyama Y, Kimura M, Asada M, Komi-Kuramochi A, Oka S, Imamura T (2008) betaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol 22:1006–1014
Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M (2007) BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA 104:7432–7437
Ito S, Kinoshita S, Shiraishi N, Nakagawa S, Sekine S, Fujimori T, Nabeshima YI (2000) Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 98:115–119
Kojima M, Kangawa K (2010) Ghrelin: from gene to physiological function. Results Probl Cell Differ 50:185–205
Egecioglu E, Stenstrom B, Pinnock SB, Tung LY, Dornonville de la Cour C, Lindqvist A, Hakanson R, Syversen U, Chen D, Dickson SL (2008) Hypothalamic gene expression following ghrelin therapy to gastrectomized rodents. Regul Pept 146:176–182
Cowley MA, Smith RG, Diano S, Tschop M, Pronchuk N, Grove KL, Strasburger CJ, Bidlingmaier M, Esterman M, Heiman ML et al (2003) The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37:649–661
Zamani N, Brown CW (2011) Emerging roles for the transforming growth factor-{beta} superfamily in regulating adiposity and energy expenditure. Endocr Rev 32:387–403
Ebendal T, Bengtsson H, Soderstrom S (1998) Bone morphogenetic proteins and their receptors: potential functions in the brain. J Neurosci Res 51:139–146
Townsend KL, Suzuki R, Huang TL, Jing E, Schulz TJ, Lee K, Taniguchi CM, Espinoza DO, McDougall LE, Zhang H et al (2012) Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB Jour Off Publ Fed Am Soc Exp Biol. doi:10.1096/fj.11-199067
Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y et al (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000–1004
Eberlein GA, Eysselein VE, Goebell H (1988) Cholecystokinin-58 is the major molecular form in man, dog and cat but not in pig, beef and rat intestine. Peptides 9:993–998
Rehfeld JF, Sun G, Christensen T, Hillingso JG (2001) The predominant cholecystokinin in human plasma and intestine is cholecystokinin-33. J Clin Endocrinol Metab 86:251–258
Moran TH, Kinzig KP (2004) Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol 286:G183–G188
Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP (1981) C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 34:154–160
Lembke V, Goebel M, Frommelt L, Inhoff T, Lommel R, Stengel A, Tache Y, Grotzinger C, Bannert N, Wiedenmann B et al (2011) Sulfated cholecystokinin-8 activates phospho-mTOR immunoreactive neurons of the paraventricular nucleus in rats. Peptides 32:65–70
Chearskul S, Delbridge E, Shulkes A, Proietto J, Kriketos A (2008) Effect of weight loss and ketosis on postprandial cholecystokinin and free fatty acid concentrations. Am J Clin Nutr 87:1238–1246
Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J (2011) Long-term persistence of hormonal adaptations to weight loss. N Engl J Med 365:1597–1604
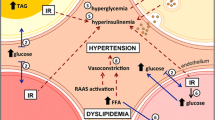
Boschmann M, Ringel J, Klaus S, Sharma AM (2001) Metabolic and hemodynamic response of adipose tissue to angiotensin II. Obes Res 9:486–491
Boschmann M, Jordan J, Schmidt S, Adams F, Luft FC, Klaus S (2002) Gender-specific response to interstitial angiotensin II in human white adipose tissue. Horm Metab Res Horm Stoffwechselforschung Horm Metab 34:726–730
Jordan J, Engeli S, Boschmann M, Weidinger G, Luft FC, Sharma AM, Kreuzberg U (2005) Hemodynamic and metabolic responses to valsartan and atenolol in obese hypertensive patients. J Hypertens 23:2313–2318
Engeli S, Schling P, Gorzelniak K, Boschmann M, Janke J, Ailhaud G, Teboul M, Massiera F, Sharma AM (2003) The adipose-tissue renin-angiotensin-aldosterone system: role in the metabolic syndrome? Int J Biochem Cell Biol 35:807–825
Boschmann M, Kreuzberg U, Engeli S, Adams F, Franke G, Klaua S, Scholze J, Weidinger G, Luft FC, Sharma AM et al (2006) The effect of oral glucose loads on tissue metabolism during angiotensin II receptor and beta-receptor blockade in obese hypertensive subjects. Horm Metab Res Horm Stoffwechselforschung Horm Metab 38:323–329
Lohn M, Kampf D, Gui-Xuan C, Haller H, Luft FC, Gollasch M (2002) Regulation of arterial tone by smooth muscle myosin type II. Am J Physiol Cell Physiol 283:C1383–C1389
Gollasch M, Dubrovska G (2004) Paracrine role for periadventitial adipose tissue in the regulation of arterial tone. Trends Pharmacol Sci 25:647–653
Gollasch M (2012) Vasodilator signals from perivascular adipose tissue. Br J Pharmacol 165:633–642
Ma L, Ma S, He H, Yang D, Chen X, Luo Z, Liu D, Zhu Z (2010) Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res Off J Jpn Soc Hypertens 33:446–453
Shioi T, McMullen JR, Tarnavski O, Converso K, Sherwood MC, Manning WJ, Izumo S (2003) Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 107:1664–1670
De Angel RE, Conti CJ, Wheatley KE, Brenner AJ, Otto G, Degraffenried LA, Hursting SD (2012) The enhancing effects of obesity on mammary tumor growth and Akt/mTOR pathway activation persist after weight loss and are reversed by RAD001. Mol Carcinog. doi:10.1002/mc.21878
Lloberas N, Cruzado JM, Franquesa M, Herrero-Fresneda I, Torras J, Alperovich G, Rama I, Vidal A, Grinyo JM (2006) Mammalian target of rapamycin pathway blockade slows progression of diabetic kidney disease in rats. J Am Soc Nephrol 17:1395–1404
Conflict of interest
The authors report no conflict of interest
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Mannaa, M., Krämer, S., Boschmann, M. et al. mTOR and regulation of energy homeostasis in humans. J Mol Med 91, 1167–1175 (2013). https://doi.org/10.1007/s00109-013-1057-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-013-1057-6