Abstract
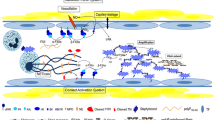
Hemostasis is a sensitive and tightly regulated process, involving vascular endothelium and blood cells, as well as factors of the coagulation and fibrinolytic cascades. In severe and invasive infectious diseases, the equilibrium between the procoagulant and anticoagulant status of the host may change dramatically and can induce life-threatening complications. A growing body of evidence suggests that the contact system, also known as the intrinsic pathway of coagulation or kallikrein/kinin system, participate in these processes. Contact activation leads to the release of the highly potent proinflammatory peptide bradykinin and initiates the intrinsic pathway of coagulation. Several studies have shown a systemic activation of the contact system in animal models of severe bacterial infections, and similar findings were also reported when monitoring patients suffering from sepsis, severe sepsis, or septic shock. Complications resulting from a systemic activation of the contact system are pathologically high levels of bradykinin, consumption of contact factors, and a subsequent induction of inflammatory reactions. These conditions may contribute to serious complications such as hypotension and vascular leakage. Here, we summarize the state of the art in this field of research with a focus on the contact system, and we also discuss a potential role for the contact system as a target for the development of novel antimicrobial strategies.

Similar content being viewed by others
References
Martin GS, Mannino DM, Eaton S, Moss M (2003) The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med 348:1546–1554
Riedemann NC, Guo RF, Ward PA (2003) Novel strategies for the treatment of sepsis. Nat Med 9:517–524. doi:10.1038/nm0503-517 nm0503-517 [pii]
Pixley RA, Zellis S, Bankes P, DeLa Cadena RA, Page JD, Scott CF, Kappelmayer J, Wyshock EG, Kelly JJ, Colman RW (1995) Prognostic value of assessing contact system activation and factor V in systemic inflammatory response syndrome. Crit Care Med 23:41–51
Hasan AA, Cines DB, Herwald H, Schmaier AH, Müller-Esterl W (1995) Mapping the cell binding site on high molecular weight kininogen domain 5. J BiolChem 270:19256–19261
Herwald H, Mörgelin M, Svensson HG, Sjöbring U (2001) Zinc-dependent conformational changes in domain D5 of high molecular mass kininogen modulate contact activation. Eur J Biochem 268:396–404
Ben Nasr AB, Herwald H, Müller-Esterl W, Björck L (1995) Human kininogens interact with M protein, a bacterial surface protein and virulence determinant. Biochem J 305:173–180
Mattsson E, Herwald H, Cramer H, Persson K, Sjöbring U, Björck L (2001) Staphylococcus aureus induces release of bradykinin in human plasma. Infect Immun 69:3877–3882
Herwald H, Dedio J, Kellner R, Loos M, Müller-Esterl W (1996) Isolation and characterization of the kininogen-binding protein p33 from endothelial cells. Identity with the gC1q receptor. J Biol Chem 271:13040–13047
Mahdi F, Shariat-Madar Z, Kuo A, Carinato M, Cines DB, Schmaier AH (2004) Mapping the interaction between high molecular mass kininogen and the urokinase plasminogen activator receptor. J Biol Chem 279:16621–16628. doi:10.1074/jbc.M313850200 M313850200 [pii]
Nakazawa Y, Joseph K, Kaplan AP (2002) Inhibition of contact activation by a kininogen peptide (HKH20) derived from domain 5. International immunopharmacology 2:1875–1885. doi:S1567-5769(02)00182-0 [pii]
Perkins R, Ngo MD, Mahdi F, Shariat-Madar Z (2008) Identification of lipopolysaccharide binding site on high molecular weight kininogen. Biochem Biophys Res Commun 366:938–943. doi:S0006-291X(07)02654-X [pii] 10.1016/j.bbrc.2007.12.042
Nordahl EA, Rydengård V, Mörgelin M, Schmidtchen A (2005) Domain 5 of high molecular weight kininogen is antibacterial. J Biol Chem 280:34832–34839
Colman RW, Jameson BA, Lin Y, Johnson D, Mousa SA (2000) Domain 5 of high molecular weight kininogen (kininostatin) down-regulates endothelial cell proliferation and migration and inhibits angiogenesis. Blood 95:543–550
Colman RW, Schmaier AH (1997) Contact system: a vascular biology modulator with anticoagulant, profibrinolytic, antiadhesive, and proinflammatory attributes. Blood 90:3819–3843
Kunapuli SP, DeLa Cadena RA, Colman RW (1993) Deletion mutagenesis of high molecular weight kininogen light chain. Identification of two anionic surface binding subdomains. J Biol Chem 268:2486–2492
Kannemeier C, Shibamiya A, Nakazawa F, Trusheim H, Ruppert C, Markart P, Song Y, Tzima E, Kennerknecht E, Niepmann M, von Bruehl ML, Sedding D, Massberg S, Günther A, Engelmann B, Preissner KT (2007) Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc Natl Acad Sci U S A 104:6388–6393. doi:0608647104 [pii] 10.1073/pnas.0608647104
Oehmcke S, Mörgelin M, Herwald H (2009) Activation of the human contact system on neutrophil extracellular traps. J Innate Immun 1:225–231
Davis AE 3rd (2008) Hereditary angioedema: a current state-of-the-art review, III: mechanisms of hereditary angioedema. Ann Allergy Asthma Immunol 100:S7–12
Frick IM, Björck L, Herwald H (2007) The dual role of the contact system in bacterial infectious disease. Thromb Haemost 98:497–502
Russell JA (2006) Management of sepsis. N Engl J Med 355:1699–1713
Rittirsch D, Flierl MA, Ward PA (2008) Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8:776–787. doi:nri2402 [pii] 10.1038/nri2402
Fourrier F, Jourdain M, Tournois A, Caron C, Goudemand J, Chopin C (1995) Coagulation inhibitor substitution during sepsis. Intensive Care Med 21(Suppl 2):S264–268
Risberg B, Andreasson S, Eriksson E (1991) Disseminated intravascular coagulation. Acta Anaesthesiol Scand Suppl 95:60–71
Levi M, Ten Cate H (1999) Disseminated intravascular coagulation. N Engl J Med 341:586–592
Mason JW, Kleeberg U, Dolan P, Colman RW (1970) Plasma kallikrein and Hageman factor in Gram-negative bacteremia. Ann Intern Med 73:545–551
Pixley RA, Colman RW (1997) The kallikrein-kinin system in sepsis syndrome. Handbook of Immunopharmacology—The Kinin System Farmer SG, ed. New York: Academic Press: 173–186
Saugstad OD, Buo L, Johansen HT, Roise O, Aasen AO (1992) Activation of the plasma kallikrein-kinin system in respiratory distress syndrome. Pediatr Res 32:431–435
Aasen AO, Smith-Erichsen N, Amundsen E (1983) Plasma kallikrein-kinin system in septicemia. Arch Surg 118:343–346
Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B (2005) Defective thrombus formation in mice lacking coagulation factor XII. J Exp Med 202:271–281. doi:jem.20050664 [pii] 10.1084/jem.20050664
Gailani D, Renne T (2007) Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol 27:2507–2513. doi:ATVBAHA.107.155952 [pii] 10.1161/ATVBAHA.107.155952
Kaufman N, Page JD, Pixley RA, Schein R, Schmaier AH, Colman RW (1991) Alpha 2-macroglobulin-kallikrein complexes detect contact system activation in hereditary angioedema and human sepsis. Blood 77:2660–2667
van Deuren M, Brandtzaeg P, van der Meer JW (2000) Update on meningococcal disease with emphasis on pathogenesis and clinical management. Clin Microbiol Rev 13:144–166
Wuillemin WA, Fijnvandraat K, Derkx BH, Peters M, Vreede W, ten Cate H, Hack CE (1995) Activation of the intrinsic pathway of coagulation in children with meningococcal septic shock. Thromb Haemost 74:1436–1441
Sriskandan S, Cohen J (2000) Kallikrein-kinin system activation in streptococcal toxic shock syndrome. Clin Infect Dis 30:961–962
Pixley RA, De La Cadena R, Page JD, Kaufman N, Wyshock EG, Chang A, Taylor FBJ, Colman RW (1993) The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. In vivo use of a monoclonal anti-factor XII antibody to block contact activation in baboons. J Clin Invest 91:61–68
Pixley RA, DeLa Cadena RA, Page JD, Kaufman N, Wyshock EG, Colman RW, Chang A, Taylor FB Jr (1992) Activation of the contact system in lethal hypotensive bacteremia in a baboon model. Am J Pathol 140:897–906
Campbell DJ (2001) The kallikrein-kinin system in humans. Clin Exp Pharmacol Physiol 28:1060–1065
Cayla C, Todiras M, Iliescu R, Saul VV, Gross V, Pilz B, Chai G, Merino VF, Pesquero JB, Baltatu OC, Bader M (2007) Mice deficient for both kinin receptors are normotensive and protected from endotoxin-induced hypotension. FASEB J 21:1689–1698. doi:fj.06-7175com [pii] 10.1096/fj.06-7175com
Morinelli TA, Webb JG, Jaffa AA, Privitera PJ, Margolius HS (2001) A metabolic fragment of bradykinin, Arg-Pro-Pro-Gly-Phe, protects against the deleterious effects of lipopolysaccharide in rats. J Pharmacol Exp Ther 296:71–76
Fischer LG, Hollmann MW, Horstman DJ, Rich GF (2000) Cyclooxygenase inhibitors attenuate bradykinin-induced vasoconstriction in septic isolated rat lungs. Anesth Analg 90:625–631
Fischer LG, Hilpert JH, Freise H, Wendholt D, Van Aken H, Sielenkamper AW (2004) Bradykinin-induced pulmonary vasoconstriction is time and inducible nitric oxide synthase dependent in a peritonitis sepsis model. Anesth Analg 99:864–871. doi:10.1213/01.ANE.0000133000.65613.F5 99/3/864 [pii]
Matsumoto K, Yamamoto T, Kamata R, Maeda H (1984) Pathogenesis of serratial infection: activation of the Hageman factor-prekallikrein cascade by serratial protease. J Biochem 96:739–749
Potempa J, Pike RN (2009) Corruption of innate immunity by bacterial proteases. J Innate Immun 1:70–87
Kalter ES, van Dijk WC, Timmerman A, Verhoef J, Bouma BN (1983) Activation of purified human plasma prekallikrein triggered by cell wall fractions of Escherichia coli and Staphylococcus aureus. J Infect Dis 148:682–691
Herwald H, Mörgelin M, Olsén A, Rhen M, Dahlbäck B, Müller-Esterl W, Björck L (1998) Activation of the contact-phase system on bacterial surfaces–a clue to serious complications in infectious diseases. Nat Med 4:298–302
Yamada T, Harber P, Pettit GW, Wing DA, Oster CN (1978) Activation of the kallikrein-kinin system in Rocky Mountain spotted fever. Ann Intern Med 88:764–768
Rapala-Kozik M, Karkowska J, Jacher A, Golda A, Barbasz A, Guevara-Lora I, Kozik A (2008) Kininogen adsorption to the cell surface of Candida spp. International immunopharmacology 8:237–241
Andersson Nordahl E, Rydengård V, Mörgelin M, Schmidtchen A (2005) Domain 5 of high molecular weight kininogen is antibacterial. J Biol Chem 280:34832–34839
Araujo RC, Kettritz R, Fichtner I, Paiva AC, Pesquero JB, Bader M (2001) Altered neutrophil homeostasis in kinin B1 receptor-deficient mice. Biol Chem 382:91–95. doi:10.1515/BC.2001.014
Frick IM, Akesson P, Herwald H, Mörgelin M, Malmsten M, Nagler DK, Björck L (2006) The contact system-a novel branch of innate immunity generating antibacterial peptides. EMBO J 25:5569–5578
Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL (2005) International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev 57:27–77. doi:57/1/27 [pii] 10.1124/pr.57.1.2
Schremmer-Danninger E, Offner A, Siebeck M, Roscher AA (1998) B1 bradykinin receptors and carboxypeptidase M are both upregulated in the aorta of pigs after LPS infusion. Biochemical and biophysical research communications 243:246–252. doi:S0006-291X(97)97999-7 [pii] 10.1006/bbrc.1997.7999
Bengtson SH, Phagoo SB, Norrby-Teglund A, Påhlman L, Mörgelin M, Zuraw BL, Leeb-Lundberg LMF, Herwald H (2006) Kinin receptor expression during Staphylococcus aureus infection. Blood 108:2055–2063
Bengtson SH, Sandén C, Mörgelin M, Marx PF, Leeb-Lundberg FLM, Meijers JCM, Herwald H (2009) Activation of TAFI on the surface of Streptococcus pyogenes evokes inflammatory reactions by modulating the kallikrein/kinin system. J Innate Immun 1:18–28
Marceau F, Regoli D (2004) Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov 3:845–852
Fein AM, Bernard GR, Criner GJ, Fletcher EC, Good JT Jr, Knaus WA, Levy H, Matuschak GM, Shanies HM, Taylor RW, Rodell TC (1997) Treatment of severe systemic inflammatory response syndrome and sepsis with a novel bradykinin antagonist, deltibant (CP-0127). Results of a randomized, double-blind, placebo-controlled trial. CP-0127 SIRS and sepsis study group. Jama 277:482–487
Merino VF, Todiras M, Campos LA, Saul V, Popova E, Baltatu OC, Pesquero JB, Bader M (2008) Increased susceptibility to endotoxic shock in transgenic rats with endothelial overexpression of kinin B(1) receptors. J Mol Med 86:791–798. doi:10.1007/s00109-008-0345-z
Nuijens JH, Eerenberg-Belmer AJ, Huijbregts CC, Schreuder WO, Felt-Bersma RJ, Abbink JJ, Thijs LG, Hack CE (1989) Proteolytic inactivation of plasma C1- inhibitor in sepsis. The Journal of clinical investigation 84:443–450
Caliezi C, Wuillemin WA, Zeerleder S, Redondo M, Eisele B, Hack CE (2000) C1-esterase inhibitor: an anti-inflammatory agent and its potential use in the treatment of diseases other than hereditary angioedema. Pharmacol Rev 52:91–112
Caliezi C, Zeerleder S, Redondo M, Regli B, Rothen HU, Zurcher-Zenklusen R, Rieben R, Devay J, Hack CE, Lammle B, Wuillemin WA (2002) C1-inhibitor in patients with severe sepsis and septic shock: beneficial effect on renal dysfunction. Crit Care Med 30:1722–1728
Hack CE, Ogilvie AC, Eisele B, Eerenberg AJ, Wagstaff J, Thijs LG (1993) C1-inhibitor substitution therapy in septic shock and in the vascular leak syndrome induced by high doses of interleukin-2. Intensive Care Med 19:S19–28
Oehmcke S, Shannon O, von Köckritz-Blickwede M, Mörgelin M, Linder A, Olin AI, Björck L, Herwald H (2009) Treatment of invasive streptococcal infection with a peptide derived from human high-molecular weight kininogen. Blood 114:444–451. doi:blood-2008-10-182527 [pii] 10.1182/blood-2008-10-182527
Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr (2001) Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 344:699–709
Acknowledgments
This work was supported in part by the foundations of Alfred Österlund, Crafoord, Greta and Johan Kock, the Blood and Defence Network and the Vascular Wall Programme at Lund University, the Medical Faculty, Lund University, the Swedish Research Council, and Hansa Medical. Hansa Medical that in part funded this study has filed patent applications on HKH20. SO and HH are listed as inventors and the applications are pending.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oehmcke, S., Herwald, H. Contact system activation in severe infectious diseases. J Mol Med 88, 121–126 (2010). https://doi.org/10.1007/s00109-009-0564-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-009-0564-y