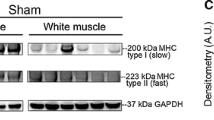
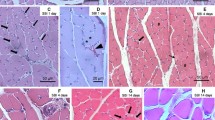
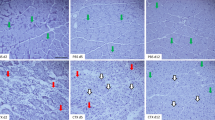
Abstract
Previous studies have suggested that an increased catabolic stage of skeletal muscle in pathological situations is mainly a reflection of ubiquitin–proteasome system-controlled proteolysis. The proteolytic mechanisms that occur after local muscle trauma are poorly defined. We investigated the effects of closed soft-tissue trauma on ubiquitin–proteasome dependent protein breakdown in rats (n = 25). The enzymatic activities of the ubiquitination and proteasome reactions were both reduced (p < 0.05) immediately after contusion of the hind limb musculus extensor digitorum longus. The same effect was observed in extracts of lung tissue from the injured animals. Cellular levels of free and protein-conjugated ubiquitin were significantly elevated upon decreased proteolytic activity. Our data support an early-state anti-proteolytic role of the ubiquitin–proteasome pathway after local injury. This further implies that there is a yet-to-be elucidated complex regulatory mechanism of muscle regeneration that involves various proteolytic systems.






Similar content being viewed by others
References
Medina R, Wing SS, Goldberg AL. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 1995;307(Pt 3):631–7.
Song XM, Ryder JW, Kawano Y, Chibalin AV, Krook A, Zierath JR. Muscle fiber type specificity in insulin signal transduction. Am J Physiol. 1999;277:R1690–6.
Goldberg AL, Tischler M, DeMartino G, Griffin G. Hormonal regulation of protein degradation and synthesis in skeletal muscle. Fed Proc. 1980;39:31–6.
Li JB, Goldberg AL. Effects of food deprivation on protein synthesis and degradation in rat skeletal muscles. Am J Physiol. 1976;231:441–8.
Reid MB. Response of the ubiquitin–proteasome pathway to changes in muscle activity. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1423–31.
Sugden PH, Fuller SJ. Regulation of protein turnover in skeletal and cardiac muscle. Biochem J. 1991;273(Pt 1):21–37.
Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin–proteasome pathway in normal and disease states. J Nutr. 1999;129:227S–37S.
Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin–proteasome pathway. N Engl J Med. 1996;335:1897–905.
Hasselgren PO, Menconi MJ, Fareed MU, Yang H, Wei W, Evenson A. Novel aspects on the regulation of muscle wasting in sepsis. Int J Biochem Cell Biol. 2005;37:2156–68.
Hasselgren PO, Wray C, Mammen J. Molecular regulation of muscle cachexia: it may be more than the proteasome. Biochem Biophys Res Commun. 2002;290:1–10.
Khal J, Wyke SM, Russell ST, Hine AV, Tisdale MJ. Expression of the ubiquitin–proteasome pathway and muscle loss in experimental cancer cachexia. Br J Cancer. 2005;93:774–80.
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51.
Voisin L, Breuille D, Combaret L, Pouyet C, Taillandier D, Aurousseau E, et al. Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+-activated, and ubiquitin–proteasome proteolytic pathways. J Clin Invest. 1996;97:1610–7.
Tisdale MJ. The ubiquitin–proteasome pathway as a therapeutic target for muscle wasting. J Support Oncol. 2005;3:209–17.
Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L. The ubiquitin–proteasome system and skeletal muscle wasting. Essays Biochem. 2005;41:173–86.
Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin–proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–19.
Larbaud D, Balage M, Taillandier D, Combaret L, Grizard J, Attaix D. Differential regulation of the lysosomal, Ca2+-dependent and ubiquitin/proteasome-dependent proteolytic pathways in fast-twitch and slow-twitch rat muscle following hyperinsulinaemia. Clin Sci (Lond). 2001;101:551–8.
Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin–proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. 2004;15:1537–45.
Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–62.
Schaser KD, Vollmar B, Menger MD, Schewior L, Kroppenstedt SN, Raschke M, et al. In vivo analysis of microcirculation following closed soft-tissue injury. J Orthop Res. 1999;17:678–85.
Tscherne H, Oestern HJ. A new classification of soft-tissue damage in open and closed fractures (author’s transl). Unfallheilkunde. 1982;85:111–5.
Ponelies N, Hirsch T, Krehmeier U, Denz C, Patel MB, Majetschak M. Cytosolic ubiquitin and ubiquitylation rates in human peripheral blood mononuclear cells during sepsis. Shock. 2005;24:20–5.
Majetschak M, Ponelies N, Hirsch T. Targeting the monocytic ubiquitin system with extracellular ubiquitin. Immunol Cell Biol. 2006;84:59–65.
Schaser KD, Bail HJ, Schewior L, Stover JF, Melcher I, Haas NP, Mittlmeier T. Acute effects of N-acetylcysteine on skeletal muscle microcirculation following closed soft tissue trauma in rats. J Orthop Res. 2005;23:231–41.
Kessler BM, Tortorella D, Altun M, Kisselev AF, Fiebiger E, Hekking BG, et al. Extended peptide-based inhibitors efficiently target the proteasome and reveal overlapping specificities of the catalytic beta-subunits. Chem Biol. 2001;8:913–29.
Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci USA. 1999;96:10403–8.
Hobler SC, Williams A, Fischer D, Wang JJ, Sun X, Fischer JE, et al. Activity and expression of the 20S proteasome are increased in skeletal muscle during sepsis. Am J Physiol. 1999;277:R434–40.
Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin–proteasome proteolysis in a model of muscle wasting. Am J Physiol. 1999;276:C1132–8.
Jagoe RT, Goldberg AL. What do we really know about the ubiquitin–proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care. 2001;4:183–90.
Mansoor O, Beaufrere B, Boirie Y, Ralliere C, Taillandier D, Aurousseau E, et al. Increased mRNA levels for components of the lysosomal, Ca2+-activated, and ATP-ubiquitin-dependent proteolytic pathways in skeletal muscle from head trauma patients. Proc Natl Acad Sci USA. 1996;93:2714–8.
Fang CH, Li BG, James JH, King JK, Evenson AR, Warden GD, Hasselgren PO. Protein breakdown in muscle from burned rats is blocked by insulin-like growth factor i and glycogen synthase kinase-3β inhibitors. Endocrinology. 2005;146:3141–9.
Seiffert M, Gosenca D, Ponelies N, Ising N, Patel MB, Obertacke U, Majetschak M. Regulation of the ubiquitin proteasome system in mechanically injured human skeletal muscle. Physiol Res. 2007;56:227–33.
Fisher BD, Baracos VE, Shnitka TK, Mendryk SW, Reid DC. Ultrastructural events following acute muscle trauma. Med Sci Sports Exerc. 1990;22:185–93.
Fisher BD, Rathgaber M. An overview of muscle regeneration following acute injury. J Phys Ther Sci. 2006;18:57–66.
Gierer P, Vollmar B, Schaser KD, Andreas C, Gradl G, Mittlmeier T. Efficiency of small-volume resuscitation in restoration of disturbed skeletal muscle microcirculation after soft-tissue trauma and haemorrhagic shock. Langenbecks Arch Surg. 2004;389:40–5.
Tjader I, Essen P, Garlick PJ, McMnurlan MA, Rooyackers O, Wernerman J. Impact of surgical trauma on human skeletal muscle protein synthesis. Clin Sci (Lond). 2004;107:601–7.
Luo JL, Hammarqvist F, Andersson K, Wernerman J. Surgical trauma decreases glutathione synthetic capacity in human skeletal muscle tissue. Am J Physiol. 1998;275:E359–65.
Farges MC, Balcerzak D, Fisher BD, Attaix D, Bechet D, Ferrara M, Baracos VE. Increased muscle proteolysis after local trauma mainly reflects macrophage-associated lysosomal proteolysis. Am J Physiol Endocrinol Metab. 2002;282:E326–35.
Tawa NE Jr, Odessey R, Goldberg AL. Inhibitors of the proteasome reduce the accelerated proteolysis in atrophying rat skeletal muscles. J Clin Invest. 1997;100:197–203.
Zhu Q, Wani G, Wang QE, El-mahdy M, Snapka RM, Wani AA. Deubiquitination by proteasome is coordinated with substrate translocation for proteolysis in vivo. Exp Cell Res. 2005;307:436–51.
Kim JH, Park KC, Chung SS, Bang O, Chung CH. Deubiquitinating enzymes as cellular regulators. J Biochem (Tokyo). 2003;134:9–18.
Combaret L, Adegoke OA, Bedard N, Baracos V, Attaix D, Wing SS. USP19 is a ubiquitin-specific protease regulated in rat skeletal muscle during catabolic states. Am J Physiol Endocrinol Metab. 2005;288:E693–700.
Fuster G, Busquets S, Almendro V, Lopez-Soriano FJ, Argiles JM. Antiproteolytic effects of plasma from hibernating bears: a new approach for muscle wasting therapy? Clin Nutr. 2007;26:658–61.
Fielding RA, Manfredi TJ, Ding W, Fiatarone MA, Evans WJ, Cannon JG. Acute phase response in exercise. III. Neutrophil and IL-1 beta accumulation in skeletal muscle. Am J Physiol. 1993;265:R166–72.
Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukoc Biol. 1999;65:492–8.
Gates C, Huard J. Management of skeletal muscle injuries in military personnel. Oper Tech Sports Med. 2005;13:247–56.
Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–38.
Acknowledgments
We thank Anja Bistron and Andreas Klusch for excellent technical help. This research was supported by the Deutsche Forschungsgemeinschaft (DFG, priority program SPP1151).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponelies, N., Gosenca, D., Ising, N. et al. Effects on the ubiquitin proteasome system after closed soft-tissue trauma in rat skeletal muscle. Eur J Trauma Emerg Surg 37, 645–654 (2011). https://doi.org/10.1007/s00068-011-0083-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00068-011-0083-8