Abstract
Purpose
Increasing evidence implicates changes in brain function following radiotherapy for head and neck cancer as precursors for brain dysfunction. These changes may thus be used as biomarkers for early detection. This review aimed to determine the role of resting-state functional magnetic resonance imaging (rs-fMRI) in detecting brain functional changes.
Methods
A systematic search was performed in the PubMed, Scopus, and Web of Science (WoS) databases in June 2022. Patients with head and neck cancer treated with radiotherapy and periodic rs-fMRI assessments were included. A meta-analysis was performed to determine the potential of rs-fMRI for detecting brain changes.
Results
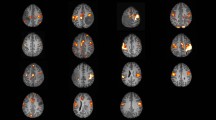
Ten studies with a total of 513 subjects (head and neck cancer patients, n = 437; healthy controls, n = 76) were included. A significance of rs-fMRI for detecting brain changes in the temporal and frontal lobes, cingulate cortex, and cuneus was demonstrated in most studies. These changes were reported to be associated with dose (6/10 studies) and latency (4/10 studies). A strong effect size (r = 0.71, p < 0.001) between rs-fMRI and brain changes was also reported, suggesting rs-fMRI’s capability for monitoring brain alterations.
Conclusion
Resting-state functional MRI is a promising tool for detecting brain functional changes following head and neck radiotherapy. These changes are correlated with latency and prescription dose.


Similar content being viewed by others
References
Li Y, Huang X, Jiang J et al (2018) Clinical variables for prediction of the therapeutic effects of bevacizumab monotherapy in nasopharyngeal carcinoma patients with radiation-induced brain necrosis. Int J Radiat Oncol Biol Phys 100:621–629. https://doi.org/10.1016/j.ijrobp.2017.11.023
Stone JB, DeAngelis LM (2016) Cancer-treatment-induced neurotoxicity—focus on newer treatments. Nat Rev Clin Oncol 13:92–105. https://doi.org/10.1038/nrclinonc.2015.152
Chen J, Dassarath M, Yin Z et al (2011) Radiation induced temporal lobe necrosis in patients with nasopharyngeal carcinoma: a review of new avenues in its management. Radiat Oncol 6:128
Ma Q, Wu D, Zeng LL et al (2016) Radiation-induced functional connectivity alterations in nasopharyngeal carcinoma patients with radiotherapy. Medicine 95:e4275. https://doi.org/10.1097/MD.0000000000004275
Ding Z, Zhang H, Lv X et al (2018) Radiation-induced brain structural and functional abnormalities in presymptomatic phase and outcome prediction. Hum Brain Mapp 39:407–427
Soussain C, Ricard D, Fike JR et al (2009) CNS complications of radiotherapy and chemotherapy. Lancet 374(9701):1639–1651. https://doi.org/10.1016/S0140-6736(09)61299-X
Karunamuni R, Bartsch H, White NS et al (2016) Dose-dependent cortical thinning after partial brain irradiation in high-grade glioma. Int J Radiat Oncol Biol Phys 94(2):297–304. https://doi.org/10.1016/j.ijrobp.2015.10.026
Seibert TM, Karunamuni R, Bartsch H et al (2017) Radiation dose-dependent hippocampal atrophy detected with longitudinal volumetric magnetic resonance imaging. Int J Radiat Oncol Biol Phys 97(2):263–269. https://doi.org/10.1016/j.ijrobp.2016.10.035
Makale MT, McDonald CR, Hattangadi-Gluth JA et al (2017) Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat Rev Neurol 13(1):52–64. https://doi.org/10.1038/nrneurol.2016.185
Gondi V, Tome WA, Mehta MP (2010) Why avoid the hippocampus? A comprehensive review. Radiother Oncol 97:370–376
Biswal B, Yetkin FZ, Haughton VM et al (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34(4):537–541
Zhang D, Raichle ME (2010) Disease and the brain’s dark energy. Nat Rev Neurol 6(1):15–28. https://doi.org/10.1038/nrneurol.2009.198
Korgaonkar MS, Ram K, Williams LM et al (2014) Establishing the resting state default mode network derived from functional magnetic resonanceimaging tasks as an endophenotype: a twins study. Hum Brain Mapp 35:3893–3902
Lv X, He H, Yang Y et al (2018) Radiation-induced hippocampal atrophy in patients with nasopharyngeal carcinoma early after radiotherapy: a longitudinal MR-based hippocampal subfield analysis. Brain Imaging Behav. https://doi.org/10.1007/s11682-018-9931-z
Yang Y, Lin X, Li J et al (2019) Aberrant brain activity at early delay stage post-radiotherapy as a biomarker for predicting neurocognitive dysfunction late-delayed in patients with nasopharyngeal carcinoma. Front Neurol 10:752. https://doi.org/10.3389/fneur.2019.00752
Damoiseaux JS, Rombouts SA, Barkhof F et al (2006) Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103:13848–13853. https://doi.org/10.1073/pnas.0601417103
Zang YF, He Y, Zhu CZ et al (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91
Goncalves SI, de Munck JC, Pouwels PJ et al (2006) Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variability. Neuroimage 30:203–213
Zang Y, Jiang T, Lu Y et al (2004) Regional homogeneity approach to fMRI data analysis. Neuroimage 22:394–400. https://doi.org/10.1016/j.neuroimage.2003.12.030
Zuo XN, Xing XX (2014) Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci Biobehav Rev 45:100–118. https://doi.org/10.1016/j.neubiorev.2014.05.009
Lv XF, Qiu YW, Tian JZ et al (2013) Abnormal regional homogeneity of resting-state brain activity in patients with HBV-related cirrhosis without overt hepatic encephalopathy. Liver Int 33:375–383. https://doi.org/10.1111/liv.12096
Qiu Y, Guo Z, Han L, et al (2017) Network-level dysconnectivity in patients with nasopharyngeal carcinoma (NPC) early post-radiotherapy: longitudinal resting state fMRI study. Brain Imaging Behav 5:1279–1289. https://doi.org/10.1007/s11682-017-9801-0
Zhao Z, Tang C, Yin D et al (2018) Frequency-specific alterations of regional homogeneity in subcortical stroke patients with different outcomes in hand function. Hum Brain Mapp 39:4373–4384. https://doi.org/10.1002/hbm.24277
Lin WC, Hsu TW, Chen CL et al (2015) Resting State-fMRI with ReHo analysis as a non-invasive modality for the prognosis of cirrhotic patients with overt hepatic encephalopathy. PLoS ONE 10:e126834. https://doi.org/10.1371/journal.pone.0126834
Zhang Y, Gao J, Zhou H et al (2019) Pre-symptomatic local brain activity and functional connectivity alterations in nasopharyngeal carcinoma patients who developed radiation encephalopathy following radiotherapy. Brain Imaging Behav. https://doi.org/10.1007/s11682-019-00145-0
Tomasi D, Volkow ND (2010) Functional connectivity density mapping. Proc Natl Acad Sci USA 107:9885–9890
Kravitz DJ, Saleem KS, Baker CI et al (2013) The ventral visual pathway: an expanded neural framework for the processing of object quality. Trends Cogn Sci 17(1):26–49. https://doi.org/10.1016/j.tics.2012.10.011
Lee AW, Law SC, Ng SH et al (1992) Retrospective analysis of nasopharyngeal carcinoma treated during 1976–1985: late complications following megavoltage irradiation. Br J Radiol 65(778):918–928. https://doi.org/10.1259/0007-1285-65-778-918
Lee AW, Ng WT, Pan JJ et al (2019) International guideline on dose prioritization and acceptance criteria in radiation therapy planning for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 105(3):567–580. https://doi.org/10.1016/j.ijrobp.2019.06.2540
Calhoun VD, Adali T, Pearlson GD et al (2001) A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14(3):140–151
Beckmann CF, DeLuca M, Devlin JT et al (2005) Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond, B, Biol Sci 360(1457):1001–1013. https://doi.org/10.1098/rstb.2005.1634
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214(5):655–667. https://doi.org/10.1007/s00429-010-0262-0
Greene-Schloesser D, Robbins ME (2012) Radiation-induced cognitive impairment—from bench to bedside. Neuro Oncol 14(4):37–44. https://doi.org/10.1093/neuonc/nos196
Zhang LY, Yang HY, Tian Y (2015) Radiation-induced cognitive impairment. Ther Targets Neurol Dis 2:e837. https://doi.org/10.14800/ttnd.837
Sporns O, Honey CJ, Kotter R (2007) Identification and classification of hubs in brain networks. PLoS ONE 2:e1049
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Liao X, Vasilakos AV, He Y (2017) Small-world human brain networks: perspectives and challenges. Neurosci Biobehav Rev 77:286–300
Wang HZ, Qiu SJ, Lv XF et al (2012) Diffusion tensor imaging and 1H-MRS study on radiation-induced brain injury after nasopharyngeal carcinoma radiotherapy. Clin Radiol 67:340–345
Xiong WF, Qiu SJ, Wang HZ et al (2013) 1H-MR spectroscopy and diffusion tensor imaging of normal-appearing temporal white matter in patients with nasopharyngeal carcinoma after irradiation: initial experience. J Magn Reson Imaging 37:101–108
Suurmond R, van Rhee H, Hak T (2017) Introduction, comparison and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods 8(4):537–553. https://doi.org/10.1002/jrsm.1260
Leng X, Qin C, Lin H et al (2021) Altered topological properties of static/dynamic functional networks and cognitive function after radiotherapy for nasopharyngeal carcinoma using resting-state fMRI. Front Neurosci 15:690743. https://doi.org/10.3389/fnins.2021.690743
Zhao LM, Kang YF, Gao JM et al (2021) Functional connectivity density for radiation encephalopathy prediction in nasopharyngeal carcinoma. Front Oncol 11:687127. https://doi.org/10.3389/fonc.2021.687127
Ma Q, Zeng LL, Qin J et al (2017) Radiation-induced cerebellar-cerebellar functional connectivity alterations in nasopharyngeal carcinoma patients. Neuroreport 28:705–711. https://doi.org/10.1097/WNR.0000000000000813
Fu G, Xie Y, Pan J et al (2022) Longitudinal study of irradiation-induced brain functional network alterations in patients with nasopharyngeal carcinoma. Radiother Oncol. https://doi.org/10.1016/j.radonc.2022.06.008
Ren WT, Li YX, Wang K et al (2019) Cerebral function abnormalities in patients with nasopharyngeal carcinoma after radiotherapy: an observational magnetic resonance resting-state study. Chin Med J. https://doi.org/10.1097/CM9.0000000000000277
Qiu Y, Guo Z, Han L et al (2018) Network-level dysconnectivity in patients with nasopharyngeal carcinoma (NPC) early post-radiotherapy: longitudinal resting state fMRI study. Brain Imaging Behav 12:1279–1289. https://doi.org/10.1007/s11682-017-9801-0
Tononi G, Sporns O, Edelman GM (1994) A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci USA 91:5033–5037
Liu Y, Gao JH, Liotti M et al (1999) Temporal dissociation of parallel processing in the human subcortical outputs. Nature 400:364–367
Shi L, Lin L (2019) The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine 98(23):e15987. https://doi.org/10.1097/MD.0000000000015987
Brown WR, Thore CR, Moody DM et al (2005) Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res 164:662–668. https://doi.org/10.1667/RR3453.1
Greene-Schloesser D, Robbins ME, Peiffer AM et al (2012) Radiation-induced brain injury: a review. Front Oncol 2:73. https://doi.org/10.3389/fonc.2012.00073
Sundgren PC (2009) MR spectroscopy in radiation injury. Ajnr Am J Neuroradiol 30:1469–1476
Chen WS, Li JJ, Zhang JH et al (2014) Magnetic resonance spectroscopic imaging of brain injury after nasopharyngeal cancer radiation in early delayed reaction. Genet Mol Res 13:6848–6854
Abayomi OK (1996) Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol 35:659–663. https://doi.org/10.3109/02841869609083995
Voon NS, Lau FN, Zakaria R et al (2021) MRI-based brain structural changes following radiotherapy of nasopharyngeal carcinoma: a systematic review. Cancer Radiother 25(1):62–71. https://doi.org/10.1016/j.canrad.2020.07.008
Rocca MA, Pravata E, Valsasina P et al (2015) Hippocampal- DMN disconnectivity in MS is related to WM lesions and depression. Hum Brain Mapp 36:5051–5063
Voon NS, Manan HA, Yahya N (2022) Diffusion tensor imaging indices as biomarkers for cognitive changes following paediatric radiotherapy: a systematic review and meta-analysis. Strahlenther Onkol. https://doi.org/10.1007/s00066-022-01905-6
Schnegg CI, Greene-Schloesser D, Kooshki M et al (2013) The PPAR delta agonist GW0742 inhibits neuroinflammation, but does not restore neurogenesis or prevent early delayed hippocampal-dependent cognitive impairment after whole-brain irradiation. Free Radic Biol Med 61:1–9
Monje ML, Palmer T (2003) Radiation injury and neurogenesis. Curr Opin Neurol 16:129–134
Hahn B, Ross TJ, Stein EA (2006) Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage 32:842–853
Cheung MC, Chan AS, Law SC et al (2003) Impact of radionecrosis on cognitive dysfunction in patients after radiotherapy for nasopharyngeal carcinoma. Cancer 97:2019–2026
Guo Z, Han L, Yang Y, He H, Li J, Chen H, Song T, Qiu Y, Lv X (2018) Longitudinal brain structural alterations in patients with nasopharyngeal carcinoma early after radiotherapy. Neuroimage Clin 19:252–259. https://doi.org/10.1016/j.nicl.2018.04.019
Newman ME (2002) Assortative mixing in networks. Phys Rev Lett 89:208701. https://doi.org/10.1103/PhysRevLett.89.208701
Ravasz E, Barabási AL (2003) Hierarchical organization in complex networks. Phys Rev E Stat Nonlin Soft Matter Phys 67:26112. https://doi.org/10.1103/PhysRevE.67.026112
Wang S, Wu EX, Qiu D et al (2009) Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Res 69(3):1190–1198. https://doi.org/10.1158/0008-5472.CAN-08-2661
Yahya N, Manan HA (2021) Diffusion tensor imaging indices to predict cognitive changes following adult radiotherapy. Eur J Cancer Care 30(1):e13329. https://doi.org/10.1111/ecc.13329
Voon NS, Manan HA, Yahya N (2021) Cognitive decline following radiotherapy of head and neck cancer: systematic review and meta-analysis of MRI correlates. Cancers (Basel) 13(24):6191. https://doi.org/10.3390/cancers13246191
Dumas JA, Makarewicz J, Schaubhut GJ et al (2013) Chemotherapy altered brain functional connectivity in women with breast cancer: a pilot study. Brain Imaging Behav 7:524–532
Hosseini SM, Kesler SR (2014) Multivariate pattern analysis of FMRI in breast cancer survivors and healthy women. J Int Neuropsychol Soc 20:391–401
Kesler SR, Wefel JS, Hosseini SM et al (2013) Default mode network connectivity distinguishes chemotherapy-treated breast cancer survivors from controls. Proc Natl Acad Sci USA 110:11600–11605
Wu L, Zhao H, Weng H et al (2019) Lasting effects of general anaesthetics on the brain in the young and elderly:’mixed picture’of neurotoxicity, neuroprotection and cognitive impairment. J Anesth 33(2):321–335
Sprung J, Schulte PJ, Knopman DS et al (2019) Cognitive function after surgery with regional or general anaesthesia: a population-based study. Alzheimers Dement 15(10):1243–1252
Manan HA, Franz EA, Yahya N (2020) Functional connectivity changes in patients with brain tumours—a systematic review on resting state-fMRI. Neurol Psychiatry Brain Res 36:73–82
Funding
This work was funded by the Ministry of Higher Learning (Malaysia)—Fundamental Research Grant (FRGS/1/2021/SS03/UKM/02/1), and the National University of Malaysia under grants FF-2020-013 and GP-2021-K017963.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, literature search, and data analysis were performed by Noor Shatirah Voon, Noorazrul Yahya, and Hanani A. Manan. The first draft of the manuscript was written by Noor Shatirah Voon and all authors commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
N.S. Voon, H.A. Manan, and N. Yahya declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Data availability statement
The data presented in this review are available in the main article or supplementary materials.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Voon, N.S., Manan, H.A. & Yahya, N. Role of resting-state functional MRI in detecting brain functional changes following radiotherapy for head and neck cancer: a systematic review and meta-analysis. Strahlenther Onkol 199, 706–717 (2023). https://doi.org/10.1007/s00066-023-02089-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-023-02089-3