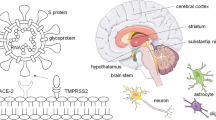
Abstract
Purpose
This systematic review is aimed at synthesising the literature base to date on the frequency and topographical distribution of neuroanatomical changes seen on imaging following COVID-19 invasion with a focus on both the acute and chronic phases of the disease.
Methods
In this study, 8 databases were systematically searched to identify relevant articles published from December 2019 to March 2022 and supplemented with a manual reference search. Data were extracted from the included studies and narrative synthesis was employed to integrate the findings.
Results
A total of 110 studies met the inclusion criteria and comprised 119,307 participants (including 31,073 acute and 143 long COVID-19 patients manifesting neurological alterations) and controls. Considerable variability in both the localisation and nature of neuroanatomical abnormalities are noted along the continuum with a wide range of neuropathologies relating to the cerebrovascular/neurovascular system, (sub)cortical structures (including deep grey and white matter structures), brainstem, and predominant regional and/or global alterations in the cerebellum with varying degrees of spinal involvement.
Conclusion
Structural regional alterations on neuroimaging are frequently demonstrated in both the acute and chronic phases of SARS-CoV‑2 infection, particularly prevalent across subcortical, prefrontal/frontal and cortico-limbic brain areas as well as the cerebrovascular/neurovascular system. These findings contribute to our understanding of the acute and chronic effects of the virus on the nervous system and has the potential to provide information on acute and long-term treatment and neurorehabilitation decisions.
Similar content being viewed by others
Introduction
The typical clinical spectrum of SARS-CoV‑2 (COVID-19) infection is widespread and encompasses asymptomatic infection, mild upper and/or lower respiratory tract illness, fever, severe viral pneumonia with respiratory failure and, in some cases, death [1]. While it was initially identified as predominantly a respiratory infection [2], COVID-19 is now widely considered a multisystemic disease, causing cardiovascular, renal, gastrointestinal, hepatic, haematological [3] and metabolic disorders [4]. Accumulating evidence has highlighted potential relationships and involvement of the central nervous system (CNS) in the invasion mechanism of the virus [5, 6] as evidenced in large cohorts of patients displaying neurological manifestations [7,8,9]. For example, in a cohort of patients hospitalised with COVID-19 (n = 214) in Wuhan, 36.4% presented with neurological symptoms, including dizziness, headache, impaired consciousness, and acute cerebrovascular events. Similarly, LaRovere et al. [10] in a large retrospective study (n = 1695) from the USA, reported several neurological complications, including loss of taste and smell, altered awareness or confusion, fatigue/weakness, headache, and seizures or status epilepticus across a large proportion (21.5%) of the cohort.
Neuroimaging studies implicated various brain regions including the involvement of the olfactory areas coupled with prefrontal and cortico-limbic structures in the pathophysiology of COVID-19 to explain these neurological manifestations [11,12,13]. A recent review of several clinical case studies also highlighted spinal involvement of COVID-19 infections, providing valuable insights into the diagnoses and management of affected patients [150]. Similar findings were recently reported in previous systematic reviews [7, 14, 151]; however, most of these studies are limited by methodological heterogeneities including the inclusion of relatively small sample studies and cases (n < 10 patients in brain studies) and irreproducible literature search strategies.
Considering the quickly evolving nature of the pandemic and mutations of the COVID-19, it is critical to comprehensively analyse the available literature to update the whole continuum of neuroanatomical (brain and spine) imaging findings relating to all phases (i.e., acute and chronic) of the disease. This systematic review aims to collate early evidence, frequency of occurrence and topographical distribution of neuroanatomical abnormalities following COVID-19 infection with a focus on acute and chronic (including possible long COVID) disease phases. The findings will provide valuable insights into expected topographical neuroimaging features post-COVID-19 infection, and possibly guide future neurological management of patients, while adding to the evolving literature base on the long-term effects of COVID-19.
Methods
Protocol and Registration
The updated version of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [15] was employed for this study. The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO ID: CRD42022315428) prior to the start of the study.
Search Strategy
The keywords required for the search were identified using the Participants, Interventions, Comparators, Outcomes, and Study (PICOS) design framework [16, 17] to guide the search and obtain the specific studies that are appropriate for the review. The terms were developed by the research team together with an expert librarian (JH) who confirmed it to be appropriate. The systematic search was conducted independently by two reviewers (CK and OAI) across key databases: PubMed (via Ovid), Scopus, ScienceDirect, EMBASE (via Ovid), PsycINFO (via Ovid), the Cochrane Library, Web of Science and CINAHL to identify relevant articles published between December 2019 and March 2022. These timepoints were selected according to the COVID-19 epidemiological trends to date [18]. The search was independently updated by two reviewers (JAA and TNA) in July 2023. The reference lists of the selected articles were hand-searched for additional studies not identified in the initial electronic search. An iterative process using controlled vocabulary, free text, synonyms, and related terms interconnected by Boolean operators (“AND” and “OR” only) was employed for the query search development. The search was conducted using the keyword combinations: COVID-19, neuroimaging, brain changes and spinal changes (Table 1).
Eligibility Criteria
In accordance with the PICOS framework, the eligibility criteria are detailed:
-
i)
Study design: case-control studies, observational cohort studies (retrospective and prospective studies), and randomised controlled trials (RCTs) were included. Case studies/series that specifically focused on the brain were included if they had a sample size ≥ 10. Considering the rarity of studies focusing on spinal changes, studies of sample size ≥ 5 were included.
-
ii)
Participants: the participants were patients with acute (or current) and long (or post) COVID-19 disease. There were no restrictions regarding age, sex, ethnicity and/or disease risk groups with a COVID-19 infection. Studies reporting on patients with COVID-19 without neuroimaging and/or neurological data were excluded.
-
iii)
Interventions: interventional and/or follow-up studies reporting structural and functional neuroanatomical changes using neuroimaging i.e., magnetic resonance imaging (MRI) and computed tomography (CT) in COVID-19 patients were included.
-
iv)
Comparators: studies reporting associations between clinical symptomatology and observed neuroanatomical changes were included.
-
v)
Outcomes: studies were eligible whose main outcomes were localisation of structural and functional neuroanatomical changes in patients with COVID-19, measured using neuroimaging methods, such as MRI and CT scans, and clinical symptomatology (including neurological and/or psychological measures) in the patients. Studies reporting changes in the brain and the spine (and associated structures) measured via CT scan, structural and/or functional magnetic resonance imaging (fMRI) scan, and hybrid imaging (e.g., positron emission tomography-computed tomography (PET-CT) after COVID-19 infection were included. Studies were also considered if they reported multiple diagnoses (e.g., both brain, spinal and other related clinical conditions), but data on the changes in brain and spinal structure or activity were explicitly collected and analysed separately. Studies employing other neuroimaging modalities, such as electroencephalogram (EEG), were excluded as the study focussed on structural measures of neuroanatomy.
In addition to the PICOS framework requirements, inclusion was limited to only articles published in English. Review articles, pictorial essays, letters to the editor, correspondence, postscript and research letters, unpublished data, commentaries, opinion papers, thesis/dissertations, conference abstracts, and other topical proceedings were excluded.
Study Selection
In the first phase of screening, two reviewers (CK and OAI) independently screened the titles and abstracts to exclude articles that were irrelevant to the systematic review. The second phase related to independent full text screening of the remaining articles that met the inclusion criteria. Disagreements were resolved by consensus and/or by consultation with the principal investigator (TNA). The screening process was undertaken using the web-based version of the Rayyan software [19].
Data Extraction
Data were collected manually via a tabular template for relevant information and recorded in Microsoft Excel 365 (Microsoft Inc, Redmond, WA, USA). The following characteristics were extracted: references and country of origin, study type, total number of participants, total number of acute and long COVID-19 patients, neuroimaging modality, neuroanatomical regions involved, and clinical findings. Two reviewers (CK and OAI) independently extracted data from the included studies and disagreements were resolved in a consensus meeting with the principal investigator (TNA).
Risk of Bias and Quality Assessment
Bias of the included studies was assessed by two independent reviewers (CK and OAI) using the Risk of Bias Assessment Tool for Nonrandomised Studies (RoBANS) [20]. Data were extracted and input into a Microsoft Excel spreadsheet by each reviewer and classified into three grades: low risk, high risk, or unclear. The outcome was evaluated by a third reviewer (JAA) and the reported discrepancies were resolved through discussion or through consultation with the research team in a consensus meeting.
Results
Literature Search Outcome and Management
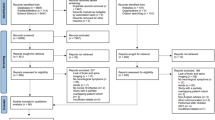
A PRISMA flowchart briefly describing the article identification, screening, and selection process is detailed (Fig. 1). A total of 8788 articles were identified through database searches. Of these, a total of 4907 remained after removal of duplicates at the end of the identification phase. Following the application of the inclusion and exclusion criteria during full text check, 4797 articles were excluded. Additional searches on ResearchGate and Google Scholar were performed for a complete list and 1 additional article was identified. The updated search included 9 relevant articles. A total of 110 articles were eligible and included for this review (Fig. 1).
Characteristics of the Included Studies
Characteristics of the included studies are briefly presented in Table 2 and in supplementary material 1. Of the 110 included studies, 76 (69.09%) were retrospective, 20 (18.18%) were prospective, 8 (7.27%) were cross-sectional, and 5 (4.55%) were observational.
Total participants reported in the included studies amounted to 119,307 (individual study sample sizes ranged from 10 to 18,407) including 31,073 acute and 143 long COVID-19 (patients manifesting neurological alterations) and controls. There was an uneven geographical distribution of the included studies in relation to study sites with 31.19% (n = 34) from United States research centres (supplementary material S1); however, no differences in neuroanatomical distribution of findings in relation to the geographical sites where the included studies were conducted were found.
Of the included articles, a proportion of studies utilised the following neuroimaging modalities: 45 (40.90%) combined brain MRI and CT, 44 (40.00%) MRI only, 17 (15.45%) CT only, 1 (0.91%) PET/MR, 1 (0.91%) PET/CT and PET/resting state functional magnetic resonance imaging (rsfMRI), and 1 (0.91%) for 18F-fluorodeoxyglucose positron emission tomography-computed tomography scan (18 FDG-PET/CT). Of note, 16.36% (n = 18) of studies comprehensively explored both the spine and the brain simultaneously (Table 2; supplementary material S1).
Risk of Bias and Quality Assessment
Figure 2 provides an overview of the six domain outcome summaries from the risk of bias assessment. The domain relating to the selection of participants in the included studies reported 59 (53.64%) studies to be of high risk of bias and 50 (45.45%) at low risk. The domain relating to confounding variables highlighted 63 (57.27%) studies as low risk of bias. Most studies (n = 84, 76.36%) recorded low risk for measurement of exposure. Of note, most studies (n = 69, 62.73%) were considered high risk for blinding of outcome assessment. For incomplete outcome data, 92 (83.64%) studies were scored as low risk of bias. For selective outcome reporting, 73 (66.36%) studies were a low risk of bias, and the risk was unclear for 36 (32.72) articles (See Table S2).
Neuroanatomical Changes from Acute Effects of SARS-CoV-2 Infection
There was considerable variability in both the localisation and nature of brain abnormalities, resulting in a wide range of neuropathologies. The commonly reported abnormalities identified on neuroimaging included cerebral ischemia, in the form of acute, subacute or chronic infarction, haemorrhage, acute strokes and persistent microhaemorrhages, cerebral venous sinus thrombosis, and supratentorial and infratentorial white matter changes.
Regional brain changes were reported in all the included studies. Of the included studies, a high incidence of neuropathology was identified in the cerebellum (41.6%), cerebrovascular/neurovascular system (39.6%), basal ganglia and thalamus (18.6%), corpus callosum (35.6%), frontal lobe (35.6%), parietal lobe (29.7%) and occipital lobe (29.7%). Other implicated regions included the motor cortex, orbitofrontal cortex, sensory cortex, temporal lobe, brainstem, amygdala, and several white matter tracts (i.e., deep/subcortical and/or nonspecific). Of note, alterations of the primary olfactory cortices were observed across almost all studies that reported on the acute effects of the infection.
Almost all (94.44%, n = 17/18) studies that reported on the spine highlighted several degrees of spinal cord involvement in the acute phase, especially in non-critical patients. However, MRI studies have reported enhancement and hyperintensity involvement in the cauda equina fibres, central cord, and nerve roots among some patients [61, 68, 70, 75, 76, 79, 86, 88, 95, 96, 104, 108, 144,145,146, 148, 149].
Regional Neuroanatomical Changes in Long COVID-19
Regional brain alterations appear to persist postinfection [37, 69, 78, 85, 111]. For example, Sollini et al. [111], in an 18F‑FDG-PET/CT study (total sample, n = 13 adult long COVID-19 patients), reported alterations in multiple regional brain networks including the primary olfactory networks (5 patients, 41.7%), involving the occipital lobe (5 patients, 41.7%), and the thalamic network (1 patient, 8.3%). In a similar 18F‑FDG-PET/CT study (total sample, n = 13 post-acute COVID-19 patients), Kiatkittikul et al. [78] reported hypometabolism in the parietal lobe (11 patients, 91.7%), temporal lobe (11 patients, 91.7%), frontal lobe (5 patients, 41.7%), occipital lobe (5 patients, 41.7%), and thalamus (1 patient, 8.3%) with general recovery and/or preservation of other regional neuroanatomical structures. In contrast to these findings, Dressing et al. [152] observed no distinct pathological findings of hypermetabolic predominance.
Findings relating to the spine were mostly of either degenerative character with other observations including demyelinated plaques, and spinal lesions while others remained unremarkable despite persistence of clinical symptoms [108, 147].
Clinical Symptomologies of Neurological Relevance Reported Across Studies and Disease Phases
Clinical findings were reported across 94 of the 101 included studies. Headache, a commonly reported neurological symptom of COVID-19 infection, was reported by 53 out of 101 (56.3%) of the included studies, followed less commonly by seizure (43.6%), encephalopathy (28.7%), (haemorrhage (23.6%), ischaemic infarcts (13.8%)) and associated strokes (23.4%) (Table 3).
Other clinical findings which were less frequently reported across the included studies are summarised (see S1 Table).
Discussion
The findings revealed considerable variability in both the localisation and nature of abnormalities detected on neuroimaging, encompassing a wide range of neuropathologies affecting the cerebrovascular/neurovascular system, basal ganglia and thalamus, corpus callosum, motor cortex, orbitofrontal lobe, sensory cortex, temporal lobe, brainstem, amygdala and predominant regional and/or global alterations in the cerebellum. Of note, alterations of the primary olfactory cortex were observed across almost all studies that reported on the acute effects of the infection. Olfactory brain network hypometabolism in long COVID-19 patients has also been noted. Along the neuroanatomical continuum to the spine, transverse myelitis, meningoencephalitis, and various degrees of inflammatory reaction along the spinal cord were noted especially in the acute phase of the disease.
Neuroanatomical Changes in the Acute Phase of the Disease
Of the included participants in the reported studies, 23% had acute COVID-19, presenting with neurological manifestations, and underwent either brain CT or MR imaging. SARS-CoV‑2 was associated with structural neuroanatomical [40] and intensity abnormalities [29, 79, 83, 112] in the olfactory bulb/tract. These deficits consisted of altered cortical volume [85], thickness [119] and hypometabolism [111, 120, 121]. Additional alterations of the primary olfactory cortex and related networks were observed across almost all studies that reported on the acute effects of the infection [11, 34, 40, 56, 93, 111, 112]. These findings underscore the importance of the olfactory system as a unique anatomical element that provides an optimal conduit for neuroinvasion [13]. In terms of symptomatology, the primary involvement of the olfactory system in the pathophysiology of the COVID-19 infection explains anosmia and in some cohorts headaches as an early marker of the SARS-CoV‑2 infection [122].
Surprisingly, the cerebellum was found to be affected in the acute stages of the disease and across most studies and case studies [8, 23, 24, 28,29,30, 39, 42, 47, 48, 50, 52, 54,55,56, 59, 60, 62, 67, 69, 70, 74,75,76, 79,80,81, 84,85,86, 89, 92, 93, 95,96,97, 100, 102, 108,109,110, 118], mostly presenting as cerebellar ataxia (for example, see [123, 124]). This was characterised by accentuation of atrophy in the cerebellum and its corresponding neural connections. SARS-CoV‑2 affects the cerebellum via direct viral invasion, but even more so through its effects on immune, haematological, and metabolic pathways [125]. The involvement of the cerebellum in the pathophysiology of COVID-19 is not fully understood; however, our findings highlight a high prevalence of involvement of this structure and calls for further investigation. Other neuroanatomical alterations were reported in acute COVID-19 across the cerebrovascular/neurovascular system, basal ganglia and thalamus, corpus callosum, regional frontal lobe, parietal lobe, and occipital lobe. The medial temporal lobe appears particularly vulnerable in the pathophysiology of COVID-19, thus resulting in cognitive deficits leading to language and memory impairments [126].
White matter abnormalities along the tracts of the olfactory cortex were among the most frequent neuroimaging abnormalities reported in patients with COVID-19 [8, 10, 23, 29, 32, 34, 36,37,38,39, 41,42,43, 45, 47,48,49, 52, 54, 55, 57, 58, 60, 62,63,64, 66,67,68,69,70, 75, 79, 81,82,83,84,85,86, 95, 96, 98,99,100,101, 103, 107,108,109, 113, 115]. This finding corroborates the observations of previous studies [13, 127, 128]. Other neuroimaging findings included ischaemic or haemorrhagic stroke [37, 56, 81], cerebral venous sinus thrombosis [21, 88, 105], and acute or subacute infarction [54, 68, 107]. Of note, Ntaios et al. demonstrated that patients with ischaemic stroke related to COVID-19 had worse functional outcomes and higher mortality than patients with ischaemic stroke and without COVID-19 [129].
Spinal cord involvement in the acute phase presented unremarkable features, especially in non-critical patients. Comprehensive MRI studies have reported hyperintense enhancement with spinal cord involvement across several cases of transverse myelitis, meningoencephalitis, and other acute inflammatory changes, characterised by oedema of the central cord and paraspinal musculature. This finding is consistent with a recent review of case studies of spinal involvement in COVID-19 infection [150].
Neuroanatomical Changes in the Chronic Phase of the Disease
In patients with long COVID-19, the basal ganglia and thalamus were predominately implicated, with hypometabolism reported regionally across the frontal, parietal and occipital lobes and related impairments emanating from the temporal lobe. Notably, neuroimaging investigations have evidenced spinal cord degenerative changes, demyelinated plaques, and spinal lesions observed among patients with persistent symptoms or long COVID [108, 147]. The persistence of symptomologies of neurological relevance in long COVID-19 patients relate to residual genetic material (i.e., ribonucleic acid) of SARS-COV‑2 in the central nervous system after the acute phase of the disease, which potentially results in neuronal loss and/or a delayed restoration of neuroanatomy [130]. Additionally, systemic inflammation following the active acute phase of the COVID-19 infection may potentially cause system level endotheliitis and consequently disrupt the blood–brain barrier [131, 132]. Moreover, it is known that systemic hyperinflammation is a leading cause of neurodegeneration and cognitive decline following regional brain alterations [133, 134]. In relation to the pathophysiology and underlying mechanism(s) of long COVID-19, Baig [135] suggested that oxidative stress and inflammation leads to weakened immunological response and incomplete virus eradication [3, 135], which explains the relative hypometabolism reported across regional cortices following clinical recovery from acute COVID-19.
It is somewhat surprising that out of 101 included studies, only 5 studies (4.95%) reported on long COVID-19 patients with persisting brain changes postrecovery. To the best of our knowledge, our review revealed a large clinical gap related to the lack of literature on long COVID-19 patients.
Locally, incidental neuroimaging changes were uncovered in patients with concurrent, recent or previous COVID-19 infection. These included acute ischaemic infarcts, presumed microhaemorrhages, atrophic changes, and white matter foci (supplement 1). While these changes were anecdotal and cannot be proven as a direct or indirect result of SARS-Cov‑2 infection, clinicians globally are likely to have seen similar nonspecific topographical changes on neuroimaging in conjunction with COVID-19, in turn complicating both accurate diagnosis and subsequent patient management. Further research to compare the incidence of these neuroimaging changes, and similar, in patients affected by COVID-19 (acute and chronic) and those unaffected would add important insight to this discussion.
Strengths and Limitations
This review used an extensive search strategy to collect relevant available evidence on associated abnormal brain and spinal regions on neuroimaging following COVID-19 infection, highlighting the clinicoradiologic findings based on neurological symptoms and neuroimaging modalities. Similarly, the study followed a rigorous method for article screening, and data extraction, and employed a standardised risk of bias assessment tool appropriate to the study designs that influenced the discussions and recommendations.
This study has some limitations that need to be considered. Firstly, by only including studies published in English, we may have excluded some valuable studies published in other languages. Secondly, in relation to the quality of the included studies, a large percentage had a high risk of bias in participant selection and blinding of outcome assessment, a low risk of bias due to incomplete outcome data, and an unclear risk of selective outcome reporting; however, the geographic distribution of the included studies is diverse and represent generalisable demographics. Thirdly, the quality of our included studies did not allow for a meta-analysis due to disparity in the findings for acute and long COVID-19 studies and the heterogeneity of the methodological designs of the included studies. Our findings should therefore be interpreted with caution considering the relatively low number of studies relating to long COVID-19.
Implications for Future Research, Policy, and Practice
A plethora of studies highlighting neuroanatomical changes following acute COVID-19, albeit little evidence is currently available in relation to long COVID-19 patients. The lack of studies on regional neuroanatomical changes in long COVID-19 requires further research to bridge this gap. Recent studies have demonstrated the need to focus a new lens on the COVID-19 pandemic and pay attention to long-term impacts of SARS-Cov‑2 infection of the brain [11, 136] in accordance with the WHO action plan to better understand the disease [137]. Drawing upon the Global Health 50/50, the African Population and Health Research Centre and the International Centre for Research on Women Statement on Global Tracking of COVID-19 [138], an in-depth understanding of how biological sex affects COVID-19 will have important implications for clinical management and mitigating strategies for this disease.
Further longitudinal studies with longer follow-ups are needed to evaluate clinical consequences (e.g., initial infection vs. reinfection, prevaccination vs. postvaccination COVID infection) and neuroabnormalities [139, 140] as well as other regional implications of neurological relevance (e.g., spinal involvement). Studies have reported the increasing adoption of machine learning techniques in the medical field due to their high accuracy [141, 142]. Therefore, future work should include machine learning algorithms to predict the impact of COVID-19 on affected brain and spinal regions [143]. As research in this area increases, future studies will be able to draw more complete neuroanatomical conclusions in patients with both acute and long COVID-19.
Conclusion
This systematic review presents evidence relating to the frequency of occurrence and topographical distribution of neuroanatomical abnormalities seen on brain and spinal imaging following COVID-19 infection across the acute and longer term phases of the disease. These findings contribute to our understanding of the acute and chronic effects of the virus on the brain and has the potential to inform acute and long-term treatment and neurorehabilitation decisions.
References
Mohanty SK, Satapathy A, Naidu MM, Mukhopadhyay S, Sharma S, Barton LM, et al. Severe acute respiratory syndrome coronavirus‑2 (SARS-CoV-2) and coronavirus disease 19 (COVID-19)—anatomic pathology perspective on current knowledge. Diagn Pathol. 2020;15:1–17. https://doi.org/10.1186/s13000-020-01017-8.
Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–5. https://doi.org/10.1001/jama.2020.12603.
Akbarialiabad H, Taghrir MH, Abdollahi A, Ghahramani N, Kumar M, Paydar S, et al. Long COVID, a comprehensive systematic scoping review. Infection. 2021; https://doi.org/10.1007/s15010-021-01666-x.
Ladopoulos T, Zand R, Shahjouei S, Chang JJ, Motte J, Charles James J, et al. COVID-19: Neuroimaging features of a pandemic. J Neuroimaging. 2021;31(2):228–43. https://doi.org/10.1111/jon.12819.
Liu B, Liu P, Dai L, Yang Y, Xie P, Tan Y, et al. Assisting scalable diagnosis automatically via CT images in the combat against COVID-19. Sci Rep. 2021;11(1):4145. https://doi.org/10.1038/s41598-021-83424-5.
Wan D, Du T, Hong W, Chen L, Que H, Lu S, et al. Neurological complications and infection mechanism of SARS-COV‑2. Sign Transduct Target Ther. 2021;6(1):406. https://doi.org/10.1038/s41392-021-00818-7.
Rastogi A, Bhaskar SMM. Incidence of white matter lesions in hospitalized COVID-19 patients: a meta-analysis. Microcirculation. 2022;29(3):e12749. https://doi.org/10.1111/micc.12749.
Kremer S, Lersy F, Anheim M, Merdji H, Schenck M, Oesterlé H, et al. Neurologic and neuroimaging findings in patients with COVID-19: a retrospective multicenter study. Neurology. 2020;95(13):e1868–e82. https://doi.org/10.1212/WNL.0000000000010112.
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–90. https://doi.org/10.1001/jamaneurol.2020.1127.
LaRovere KL, Riggs BJ, Poussaint TY, Young CC, Newhams MM, Maamari M, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78(5):536–47. https://doi.org/10.1001/jamaneurol.2021.0504.
Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, et al. SARS-CoV‑2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. https://doi.org/10.1038/s41586-022-04569-5.
Guerrero JI, Barragán LA, Martínez JD, Montoya JP, Peña A, Sobrino FE, et al. Central and peripheral nervous system involvement by COVID-19: a systematic review of the pathophysiology, clinical manifestations, neuropathology, neuroimaging, electrophysiology, and cerebrospinal fluid findings. BMC Infect Dis. 2021;21(1):515. https://doi.org/10.1186/s12879-021-06185-6.
Najt P, Richards HL, Fortune DG. Brain imaging in patients with COVID-19: a systematic review. Brain Behav Immun. 2021;16:100290. https://doi.org/10.1016/j.bbih.2021.100290.
Javed A. Neurological associations of SARS-CoV‑2 infection: a systematic review. CNS Neurol Disord Drug Targets. 2022;21(3):246–58. https://doi.org/10.2174/1871527320666210216121211.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic reviews. 2021;10(1):1–11. https://doi.org/10.1136/bmj.n71.
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14(1):1–10. https://doi.org/10.1186/s12913-014-0579-0.
Pollock A, Berge E. How to do a systematic review. Int J Stroke. 2018;13(2):138–56. https://doi.org/10.1177/1747493017743796.
Zhou G, Chen S, Chen Z. Advances in COVID-19: the virus, the pathogenesis, and evidence-based control and therapeutic strategies. Front Med. 2020;14(2):117–25. https://doi.org/10.1007/s11684-020-0773-x.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—A web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10. https://doi.org/10.1186/s13643-016-0384-4.
Choi Y, Lee MK. Neuroimaging findings of brain MRI and CT in patients with COVID-19: a systematic review and meta-analysis. Eur J Radiol. 2020;133:109393. https://doi.org/10.1016/j.ejrad.2020.109393.
Abdelzaher A, AlQatam M, Alsarraf L, Beheiri MH, Shehata SF, Elsebaie NA. Neuroimaging findings in hospitalized patients with COVID-19. Egypt J Radiol Nucl Med. 2022;53(1):22. https://doi.org/10.1186/s43055-022-00698-z.
Abenza-Abildúa M, Ramírez-Prieto M, Moreno-Zabaleta R, Arenas-Valls N, Salvador-Maya M, Algarra-Lucas C, et al. Neurological complications in critical patients with COVID-19. Neurologia. 2020;35(9):621–7. https://doi.org/10.1016/j.nrl.2020.07.014.
Agarwal S, Jain R, Dogra S, Krieger P, Lewis A, Nguyen V, et al. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke. 2020;51(9):2649–55. https://doi.org/10.1161/STROKEAHA.120.030940.
Agarwal S, Melmed K, Dogra S, Jain R, Conway J, Galetta S, et al. Increase in ventricle size and the evolution of white matter changes on serial imaging in critically ill patients with COVID-19. Neurocrit Care. 2021; https://doi.org/10.1007/s12028-021-01207-2.
Al-Mufti F, Amuluru K, Sahni R, Bekelis K, Karimi R, Ogulnick J, et al. Cerebral venous thrombosis in COVID-19: a New York metropolitan cohort study. AJNR Am J Neuroradiol. 2021;42(7):1196–200. https://doi.org/10.3174/ajnr.A7134.
Alonazi B, Farghaly AM, Mostafa MA, Al-Watban JA, Zindani SA, Altaimi F, et al. Brain MRI in SARS-CoV‑2 pneumonia patients with newly developed neurological manifestations suggestive of brain involvement. Sci Rep. 2021;11(1):1–9. https://doi.org/10.1038/s41598-021-00064-5.
Altunisik E, Baykan A, Sahin S, Aydin E, Erturk S. Quantitative analysis of the olfactory system in COVID-19: an MR imaging study. AJNR Am J Neuroradiol. 2021;42(12):2207–14. https://doi.org/10.3174/ajnr.A7278.
Alves VPV, Altoé A, Veloso V, Ferreira CLS, Ventura N, Corrêa DG. Computed tomography features of cerebrovascular complications in intensive care unit patients with severe COVID-19. Radiol Bras. 2021;54:283–8. https://doi.org/10.1590/0100-3984.2021.0023.
Aragao M, Leal M, Andrade PHP, Cartaxo Filho OQ, Aragao LV, Fonseca TM, et al. Clinical and radiological profiles of COVID-19 patients with neurological symptomatology: a comparative study. Viruses. 2021;13(5):845. https://doi.org/10.3390/v13050845.
Arandela K, Samudrala S, Abdalkader M, Anand P, Daneshmand A, Dasenbrock H, et al. Reversible cerebral vasoconstriction syndrome in patients with coronavirus disease: a multicenter case series. J Stroke Cerebrovasc Dis. 2021;30(12):106118. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106118.
Arica-Polat B, Gündoğdu AA, Çınar N, Uncu G, Ayas Z, İşeri P, et al. Evaluation of cognitive deficits in patients infected with COVID-19. Eur Rev Med Pharmacol Sci. 2022; https://doi.org/10.26355/eurrev_202201_27894.
Azab MA, Azzam AY, Salem AE, Reda A, Hassanein SF, Sabra M, et al. Neurological problems in the context of COVID-19 infection in Egypt. A multicenter retrospective analysis. Interdiscip Neurosurg. 2021;26:101345. https://doi.org/10.1016/j.inat.2021.101345.
Bruce SS, Kahan J, Huq T, Santillan A, Navi BB, Merkler AE, et al. Missed cerebrovascular events during prolonged sedation for COVID-19 pneumonia. J Clin Neurosci. 2021;86:180–3. https://doi.org/10.1016/j.jocn.2021.01.008.
Bungenberg J, Humkamp K, Hohenfeld C, Rust MI, Ermis U, Dreher M, et al. Long COVID-19: objectifying most self-reported neurological symptoms. Ann Clin Transl Neurol. 2022;9(2):141–54. https://doi.org/10.1002/acn3.51496.
Burulday V, Sayar MS, Bayar Muluk N. Peripheral and central smell regions in COVID-19 positive patients: an MRI evaluation. Acta Radiol. 2022;63(9):1233–42. https://doi.org/10.1177/02841851211034043.
Büttner L, Bauknecht HC, Fleckenstein FN, Kahn J, Tietze A, Bohner G, et al. Neuroimaging findings in conjunction with severe COVID-19. Rofo. 2021;193(07):822–9. https://doi.org/10.1055/a-1345-9784.
Chammas A, Bund C, Lersy F, Brisset J‑C, Ardellier F‑D, Kremer S, et al. Collicular hyperactivation in patients with COVID-19: a new finding on brain MRI and PET/CT. AJNR Am J Neuroradiol. 2021;42(8):1410. https://doi.org/10.3174/ajnr.A7158.
Chougar L, Shor N, Weiss N, Galanaud D, Leclercq D, Mathon B, et al. Retrospective observational study of brain MRI findings in patients with acute SARS-CoV‑2 infection and neurologic manifestations. Radiology. 2020;297(3):E313–E23. https://doi.org/10.1148/radiol.2020202422.
Conklin J, Frosch MP, Mukerji SS, Rapalino O, Maher MD, Schaefer PW, et al. Susceptibility-weighted imaging reveals cerebral microvascular injury in severe COVID-19. J Neurol Sci. 2021;421:117308. https://doi.org/10.1016/j.jns.2021.117308.
Coolen T, Lolli V, Sadeghi N, Rovai A, Trotta N, Taccone FS, et al. Early postmortem brain MRI findings in COVID-19 non-survivors. Neurology. 2020;95(14):e2016–e27. https://doi.org/10.1212/WNL.0000000000010116.
D’Amore F, Vinacci G, Agosti E, Cariddi L, Terrana A, Vizzari F, et al. Pressing issues in COVID-19: probable cause to seize SARS-CoV‑2 for its preferential involvement of posterior circulation manifesting as severe posterior reversible encephalopathy syndrome and posterior strokes. AJNR Am J Neuroradiol. 2020;41(10):1800–3. https://doi.org/10.3174/ajnr.A6679.
Deeb A, Kumar PC, Sakrani N, Trehan RK, Papinenei VR. Neurological presentations of COVID-19: characteristic features in a case series of hospitalized patients from Abu Dhabi, UAE. Biomed Res Int. 2021; https://doi.org/10.1155/2021/5822259.
Delorme C, Houot M, Rosso C, Carvalho S, Nedelec T, Maatoug R, et al. The wide spectrum of COVID-19 neuropsychiatric complications within a multidisciplinary centre. Brain Commun. 2021;3(3):fcab135. https://doi.org/10.1093/braincomms/fcab135.
Dilber B, Aydın ZGG, Yeşilbaş O, Sağ E, Aksoy NK, Gündoğmuş F, et al. Neurological manifestations of pediatric acute COVID infections: a single center experience. J Trop Pediatr. 2021;67(3):fmab62. https://doi.org/10.1093/tropej/fmab062.
Dixon L, McNamara C, Gaur P, Mallon D, Coughlan C, Tona F, et al. Cerebral microhaemorrhage in COVID-19: a critical illness related phenomenon? Stroke Vasc Neurol. 2020;5(4):e652. https://doi.org/10.1136/svn-2020-000652.
Dodd WS, Jabbour PM, Sweid A, Tjoumakaris S, Gooch MR, Al Saiegh F, et al. Aneurysmal subarachnoid hemorrhage in patients with coronavirus disease 2019 (COVID-19): a case series. World Neurosurg. 2021;153:e259–e64. https://doi.org/10.1016/j.wneu.2021.06.092.
Duan K, Premi E, Pilotto A, Cristillo V, Benussi A, Libri I, et al. Alterations of frontal-temporal gray matter volume associate with clinical measures of older adults with COVID-19. Neurobiol Stress. 2021;14:100326. https://doi.org/10.1016/j.ynstr.2021.100326.
Elizondo EFM, Ramírez JAV, Aguirre GB, Medina PMD, Estens JB. Central nervous system injury in patients with severe acute respiratory syndrome Coronavirus 2: MRI findings. Cureus. 2021;13:9. https://doi.org/10.7759/cureus.18052.
Ermis U, Rust MI, Bungenberg J, Costa A, Dreher M, Balfanz P, et al. Neurological symptoms in COVID-19: a cross-sectional monocentric study of hospitalized patients. Neurol Res Pract. 2021;3(1):1–12. https://doi.org/10.1186/s42466-021-00116-1.
Escalard S, Chalumeau V, Escalard C, Redjem H, Delvoye F, Hébert S, et al. Early brain imaging shows increased severity of acute ischemic strokes with large vessel occlusion in COVID-19 patients. Stroke. 2020;51(11):3366–70. https://doi.org/10.1161/STROKEAHA.120.031011.
Eskandar EN, Altschul DJ, de la Garza Ramos R, Cezayirli P, Unda SR, Benton J, et al. Neurologic syndromes predict higher in-hospital mortality in COVID-19. Neurology. 2021;96(11):e1527–e38. https://doi.org/10.1212/wnl.0000000000011356.
Fällmar D, Rostami E, Kumlien E, Ashton NJ, Jackmann S, Pavel R, et al. The extent of neuroradiological findings in COVID-19 shows correlation with blood biomarkers, Glasgow coma scale score and days in intensive care. J Neuroradiol. 2021; https://doi.org/10.1016/j.neurad.2021.11.003.
Flores-Silva FD, García-Grimshaw M, Valdés-Ferrer SI, Vigueras-Hernández AP, Domínguez-Moreno R, Tristán-Samaniego DP, et al. Neurologic manifestations in hospitalized patients with COVID-19 in Mexico City. PLoS ONE. 2021;16(4):e247433. https://doi.org/10.1371/journal.pone.0247433.
Franceschi A, Arora R, Wilson R, Giliberto L, Libman R, Castillo M. Neurovascular complications in COVID-19 infection: case series. AJNR Am J Neuroradiol. 2020;41(9):1632–40. https://doi.org/10.3174/ajnr.A6655.
Freeman CW, Masur J, Hassankhani A, Wolf RL, Levine JM, Mohan S. Coronavirus disease (COVID-19)-related disseminated leukoencephalopathy: a retrospective study of findings on brain MRI. AJR Am J Roentgenol. 2021;216(4):1046–7. https://doi.org/10.2214/AJR.20.24364.
Garcia MA, Barreras PV, Lewis A, Pinilla G, Sokoll LJ, Kickler T, et al. Cerebrospinal fluid in COVID-19 neurological complications: neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm. J Neurol Sci. 2021;427:117517. https://doi.org/10.1016/j.jns.2021.117517.
Garcia-Azorin D, Sierra Á, Trigo J, Alberdi A, Blanco M, Calcerrada I, et al. Frequency and phenotype of headache in covid-19: a study of 2194 patients. Sci Rep. 2021;11(1):1–10. https://doi.org/10.1038/s41598-021-94220-6.
Gogu AE, Motoc AG, Stroe AZ, Docu Axelerad A, Docu Axelerad D, Pârv F, et al. Clinical spectrum and neuroimagistic features in hospitalized patients with neurological disorders and concomitant coronavirus-19 infection. Brain Sci. 2021;11(9):1138. https://doi.org/10.3390/brainsci11091138.
Gorgulu U, Bayındır H, Bektas H, Kayipmaz A, San I. Coexistence of neurological diseases with Covid-19 pneumonia during the pandemic period. J Clin Neurosci. 2021;91:237–42. https://doi.org/10.1016/j.jocn.2021.06.041.
Greenway MR, Erben Y, Huang JF, Siegel JL, Lamb CJ, Badi MK, et al. Yield of head imaging in ambulatory and hospitalized patients with SARS-CoV-2: a multi-center study of 8675 patients. Neurohospitalist. 2021;11(3):221–8. https://doi.org/10.1177/1941874420980622.
Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2021;268:751–7. https://doi.org/10.1007/s00415-020-10108-x.
Günbey HP, Rona G, Karaoysal ÖA, Batirel A, Barut BÖ. Correlation of neuroimaging and thorax CT findings in patients with COVID-19: a large single-center experience. Turk J Med Sci. 2021;51(6):2850–60. https://doi.org/10.3906/sag-2105-138.
Hazzaa NM. Neurological complications associated with coronavirus disease-2019 (COVID-19): MRI features. Heliyon. 2021;7(8):e7879. https://doi.org/10.1016/j.heliyon.2021.e07879.
Hellgren L, Thornberg UB, Samuelsson K, Levi R, Divanoglou A, Blystad I. Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: an observational cohort study. BMJ Open. 2021;11(10):e55164. https://doi.org/10.1136/bmjopen-2021-055164.
Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martín Ó, Barrena C, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143(10):3089–103. https://doi.org/10.1093/brain/awaa239.
Iqbal Y, Alabdulla M, Latoo J, Kumar R, Albrahim S, Wadoo O, et al. Mania and hypomania associated with COVID-19: a series of 15 cases seen by the consultation-liaison psychiatry service in Qatar. Qatar Med J. 2021;2021(3):65. https://doi.org/10.5339/qmj.2021.65.
Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, et al. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. https://doi.org/10.1016/j.jns.2020.116923.
Jegatheeswaran V, Chan MWK, Chakrabarti S, Fawcett A, Chen YA. Neuroimaging findings of hospitalized Covid-19 patients: a Canadian retrospective observational study. Can Assoc Radiol J. 2022;73(1):179–86. https://doi.org/10.1177/08465371211002815.
Jensen-Kondering U, Neumann A, Margraf NG, Goevert F, Brueggemann N, Schunk D, et al. Cerebral imaging in patients with COVID-19 and neurological symptoms: first experience from two university hospitals in Northern Germany. Neuroradiology. 2021;193(06):667–71. https://doi.org/10.1055/a-1265-7209.
Kalekar T, Thakker V, Bansal A. Role of neuroimaging in COVID 19 infection—a retrospective study. J Radiol Nurs. 2021;40(4):370–6. https://doi.org/10.1016/j.jradnu.2021.09.003.
Kandemirli SG, Altundag A, Yildirim D, Sanli DET, Saatci O. Olfactory bulb MRI and paranasal sinus CT findings in persistent COVID-19 anosmia. Acad Radiol. 2021;28(1):28–35. https://doi.org/10.1016/j.acra.2020.10.006.
Karvigh SA, Vahabizad F, Banihashemi G, Sahraian MA, Gheini MR, Eslami M, et al. Ischemic stroke in patients with COVID-19 disease: a report of 10 cases from Iran. Cerebrovasc Dis. 2021;50(2):239–44. https://doi.org/10.1159/000513279.
Katz JM, Libman RB, Wang JJ, Sanelli P, Filippi CG, Gribko M, et al. Cerebrovascular complications of COVID-19. Stroke. 2020;51(9):e227–e31. https://doi.org/10.1161/STROKEAHA.120.031265.
Keller E, Brandi G, Winklhofer S, Imbach LL, Kirschenbaum D, Frontzek K, et al. Large and small cerebral vessel involvement in severe COVID-19: detailed clinical workup of a case series. Stroke. 2020;51(12):3719–22. https://doi.org/10.1161/STROKEAHA.120.031224.
Kelsch RD, Silbergleit R, Krishnan A. Neuroimaging in the first 6 weeks of the COVID-19 pandemic in an 8‑hospital campus: observations and patterns in the brain, head and neck, and spine. J Comput Assist Tomogr. 2021;45:592–9. https://doi.org/10.1097/RCT.0000000000001179.
Khedr EM, Abo-Elfetoh N, Deaf E, Hassan HM, Amin MT, Soliman RK, et al. Surveillance study of acute neurological manifestations among 439 Egyptian patients with COVID-19 in Assiut and Aswan university hospitals. Neuroepidemiology. 2021;55(2):109–18. https://doi.org/10.1159/000513647.
Khedr EM, Soliman RK, Abo-Elfetof N, Amin M, Mansour OY, Aly A, et al. Clinical and radiological characteristics of acute cerebrovascular diseases among Egyptian patients with COVID-19 in upper Egypt. Front Neurol. 2021;12:635856. https://doi.org/10.3389/fneur.2021.635856.
Kiatkittikul P, Promteangtrong C, Kunawudhi A, Siripongsatian D, Siripongboonsitti T, Ruckpanich P, et al. Abnormality pattern of F‑18 FDG PET whole body with functional MRI brain in post-acute COVID-19. Nucl Med Mol Imaging. 2022;56(1):29–41. https://doi.org/10.1007/s13139-021-00730-6.
Klironomos S, Tzortzakakis A, Kits A, Öhberg C, Kollia E, Ahoromazdae A, et al. Nervous system involvement in coronavirus disease 2019: results from a retrospective consecutive neuroimaging cohort. Radiology. 2020;297(3):E324–E34. https://doi.org/10.1148/radiol.2020202791.
Kulkarni R, Pujari SS, Gupta D, Ojha P, Dhamne M, Bolegave V, et al. Cerebrovascular involvement in mucormycosis in COVID-19 pandemic. J Stroke Cerebrovasc Dis. 2022;31(2):106231. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.106231.
Lambrecq V, Hanin A, Munoz-Musat E, Chougar L, Gassama S, Delorme C, et al. Association of clinical, biological, and brain magnetic resonance imaging findings with electroencephalographic findings for patients with COVID-19. JAMA Netw Open. 2021;4(3):e211489. https://doi.org/10.1001/jamanetworkopen.2021.1489.
Lersy F, Willaume T, Brisset J‑C, Collange O, Helms J, Schneider F, et al. Critical illness-associated cerebral microbleeds for patients with severe COVID-19: etiologic hypotheses. J Neurol. 2021;268:2676–84. https://doi.org/10.1007/s00415-020-10313-8.
Lin E, Lantos J, Strauss S, Phillips C, Campion T, Navi B, et al. Brain imaging of patients with COVID-19: findings at an academic institution during the height of the outbreak in New York City. AJNR Am J Neuroradiol. 2020;41(11):2001–8. https://doi.org/10.3174/ajnr.A6793.
Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, et al. Neuroimaging manifestations in children with SARS-CoV‑2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. 2021;5(3):167–77. https://doi.org/10.1016/S2352-4642(20)30362-X.
Lu Y, Li X, Geng D, Mei N, Wu P‑Y, Huang C‑C, et al. Cerebral micro-structural changes in COVID-19 patients—An MRI-based 3‑month follow-up study. EClinicalMedicine. 2020;25:100484. https://doi.org/10.1016/j.eclinm.2020.100484.
Mahammedi A, Ramos A, Bargalló N, Gaskill M, Kapur S, Saba L, et al. Brain and lung imaging correlation in patients with COVID-19: could the severity of lung disease reflect the prevalence of acute abnormalities on neuroimaging? A global multicenter observational study. AJNR Am J Neuroradiol. 2021;42(6):1008–16. https://doi.org/10.3174/ajnr.A7072.
Marcic L, Marcic M, Kojundzic SL, Marcic B, Capkun V, Vukojevic K. Personalized approach to patient with MRI brain changes after SARS-CoV‑2 infection. J Pers Med. 2021;11(6):442. https://doi.org/10.3390/jpm11060442.
Mekkawy DA, Hamdy S, Abdel-Naseer M, Shehata HS, Halfawy AA, Shalaby NM, et al. Neurological manifestations in a cohort of Egyptian patients with COVID-19: a prospective, multicenter, observational study. Brain Sci. 2022;12(1):74. https://doi.org/10.3390/brainsci12010074.
Meppiel E, Peiffer-Smadja N, Maury A, Bekri I, Delorme C, Desestret V, et al. Neurologic manifestations associated with COVID-19: a multicentre registry. Clin Microbiol Infect. 2021;27(3):458–66. https://doi.org/10.1016/j.cmi.2020.11.005.
Metwally MI, Mobashir M, Sweed AH, Mahmoud SM, Hassan AG, ElKashishy K, et al. Post COVID-19 head and neck mucormycosis: MR imaging spectrum and staging. Acad Radiol. 2022;29(5):674–84. https://doi.org/10.1016/j.acra.2021.12.007.
Naval-Baudin P, Rodriguez Caamano I, Rubio-Maicas C, Pons-Escoda A, Fernandez Vinas MM, Nunez A, et al. COVID-19 and ischemic stroke: clinical and neuroimaging findings. J Neuroimaging. 2021;31(1):62–6. https://doi.org/10.1111/jon.12790.
Nawabi J, Morotti A, Wildgruber M, Boulouis G, Kraehling H, Schlunk F, et al. Clinical and imaging characteristics in patients with SARS-CoV‑2 infection and acute intracranial hemorrhage. J Clin Med. 2020;9(8):2543. https://doi.org/10.3390/jcm9082543.
Niesen M, Trotta N, Noel A, Coolen T, Fayad G, Leurkin-Sterk G, et al. Structural and metabolic brain abnormalities in COVID-19 patients with sudden loss of smell. Eur J Nucl Med Mol Imaging. 2021;48(6):1890–901. https://doi.org/10.1007/s00259-020-05154-6.
Orman G, Desai N, Kralik S, Meoded A, Seghers V, Annapragada A, et al. Neuroimaging offers low yield in children positive for SARS-CoV‑2. AJNR Am J Neuroradiol. 2021;42(5):951–4. https://doi.org/10.3174/ajnr.A7022.
Palabiyik F, Akcay N, Sevketoglu E, Hatipoglu N, Sari EE, Inci E. Imaging of multisystem inflammatory disease in children (MIS-C) associated with COVID-19. Acad Radiol. 2021;28(9):1200–8. https://doi.org/10.1016/j.acra.2021.05.030.
Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–20. https://doi.org/10.1093/brain/awaa240.
Pons-Escoda A, Naval-Baudín P, Majós C, Camins A, Cardona P, Cos M, et al. Neurologic involvement in COVID-19: cause or coincidence? A neuroimaging perspective. AJNR Am J Neuroradiol. 2020;41(8):1365–9. https://doi.org/10.3174/ajnr.A6627.
Qin Y, Wu J, Chen T, Li J, Zhang G, Wu D, et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021; https://doi.org/10.1172/JCI147329.
Radmanesh A, Derman A, Lui YW, Raz E, Loh JP, Hagiwara M, et al. COVID-19-associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297(1):E223–E7. https://doi.org/10.1148/radiol.2020202040.
Rapalino O, Pourvaziri A, Maher M, Jaramillo-Cardoso A, Edlow B, Conklin J, et al. Clinical, imaging, and lab correlates of severe COVID-19 leukoencephalopathy. AJNR Am J Neuroradiol. 2021;42(4):632–8. https://doi.org/10.3174/ajnr.A6966.
Rehmani R, Segan S, Maddika SR, Lei YW, Broka A. Spectrum of neurologic & neuroimaging manifestation in COVID-19. Brain Behav Immun. 2021;13:100238. https://doi.org/10.1016/j.bbih.2021.100238.
Remsik J, Wilcox JA, Babady NE, McMillen TA, Vachha BA, Halpern NA, et al. Inflammatory leptomeningeal cytokines mediate COVID-19 neurologic symptoms in cancer patients. Cancer Cell. 2021;39(2):276–83. https://doi.org/10.1016/j.ccell.2021.01.007.
Rhally A, Griffa A, Kremer S, Uginet M, Breville G, Stancu P, et al. C‑reactive protein and white matter microstructural changes in COVID-19 patients with encephalopathy. J Neural Transm. 2021;128:1899–906. https://doi.org/10.1007/s00702-021-02429-6.
Rifino N, Censori B, Agazzi E, Alimonti D, Bonito V, Camera G, et al. Neurologic manifestations in 1760 COVID-19 patients admitted to Papa Giovanni XXIII hospital, Bergamo, Italy. J Neurol. 2021;268(7):2331–8. https://doi.org/10.1007/s00415-020-10251-5.
Rouyer O, Pierre-Paul IN, Balde AT, Jupiter D, Bindila D, Geny B, et al. High prevalence of deep venous thrombosis in non-severe COVID-19 patients hospitalized for a neurovascular disease. Cerebrovasc Dis Extra. 2020;10(3):174–80. https://doi.org/10.1159/000513295.
Sabayan B, Moghadami M, Assarzadegan F, Komachali SH‑A, Poorsaadat L, Babaeepour Z, et al. COVID-19 respiratory illness and subsequent cerebrovascular events, the initial Iranian experience. J Stroke Cerebrovasc Dis. 2021;30(1):105454. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105454.
Saleh RA, Shaban E. COVID-19 neurological manifestations: correlation of cerebral MRI imaging and lung imaging—observational study. Egypt J Radiol Nucl Med. 2021;52(1):1–11. https://doi.org/10.1186/s43055-021-00630-x.
Sandoval F, Julio K, Mendez G, Valderas C, Echeverria AC, Perinetti MJ, et al. Neurologic features associated with SARS-CoV‑2 infection in children: a case series report. J Child Neurol. 2021;36(10):853–66. https://doi.org/10.1177/0883073821989164.
Sawlani V, Scotton S, Nader K, Jen J, Patel M, Gokani K, et al. COVID-19-related intracranial imaging findings: a large single-centre experience. Clin Radiol. 2021;76(2):108–16. https://doi.org/10.1016/j.crad.2020.09.002.
Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L. Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg. 2020;141:e437–e46. https://doi.org/10.1016/j.wneu.2020.05.192.
Sollini M, Morbelli S, Ciccarelli M, Cecconi M, Aghemo A, Morelli P, et al. Long COVID hallmarks on [18F] FDG-PET/CT: a case-control study. Eur J Nucl Med Mol Imaging. 2021;48(10):3187–97. https://doi.org/10.1007/s00259-021-05294-3.
Strauss S, Lantos J, Heier L, Shatzkes D, Phillips C. Olfactory bulb signal abnormality in patients with COVID-19 who present with neurologic symptoms. AJNR Am J Neuroradiol. 2020;41(10):1882–7. https://doi.org/10.3174/ajnr.A6751.
Triay R, Buchhanolla P, Gaudet A, Winter V, Gaudet A, Faraji M, Gonzalez-Toledo E, Siddaiah H, Cuellar-Saenz HH, Bailey S, et al. The Spectrum of Acute Cerebrovascular Disease in Patients with COVID-19. Biomedicines 2022;10:435. https://doi.org/10.3390/biomedicines10020435.
Tuma RL, Guedes BF, Carra R, Iepsen B, Rodrigues J, Camelo-Filho AE, et al. Clinical, cerebrospinal fluid, and neuroimaging findings in COVID-19 encephalopathy: a case series. Neurol Sci. 2021;42:479–89. https://doi.org/10.1007/s10072-020-04946-w.
Uginet M, Breville G, Assal F, Lövblad KO, Vargas MI, Pugin J, et al. COVID-19 encephalopathy: clinical and neurobiological features. J Med Virol. 2021;93(7):4374–81. https://doi.org/10.1002/jmv.26973.
Xiong W, Mu J, Guo J, Lu L, Liu D, Luo J, et al. New onset neurologic events in people with COVID-19 in 3 regions in China. Neurology. 2020;95(11):e1479–e87. https://doi.org/10.1212/WNL.0000000000010034.
Yadav T, Tiwari S, Gupta A, Garg PK, Khera PS, Rajagopal R, et al. Magnetic resonance imaging in coronavirus disease-2019 associated rhino-orbital-cerebral mucormycosis (CA-ROCM)-imaging analysis of 50 consecutive patients. Curr Probl Diagn Radiol. 2022;51(1):112–20. https://doi.org/10.1067/j.cpradiol.2021.09.004.
Yoon B, Buch K, Lang M, Applewhite B, Li M, Mehan W, et al. Clinical and neuroimaging correlation in patients with COVID-19. AJNR Am J Neuroradiol. 2020;41(10):1791–6. https://doi.org/10.3174/ajnr.A6717.
Crunfli F, Carregari VC, Veras FP, Silva LS, Nogueira MH, Antunes ASLM, et al. Morphological, cellular, and molecular basis of brain infection in COVID-19 patients. Proc Natl Acad Sci U S A. 2022;119(35):e2200960119. https://doi.org/10.1073/pnas.2200960119.
Guedj E, Campion J, Dudouet P, Kaphan E, Bregeon F, Tissot-Dupont H, et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48(9):2823–33. https://doi.org/10.1007/s00259-021-05215-4.
Kas A, Soret M, Pyatigoskaya N, Habert M‑O, Hesters A, Le Guennec L, et al. The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging. 2021;48(8):2543–57. https://doi.org/10.1007/s00259-022-05812-x.
Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem Senses. 2021; https://doi.org/10.1093/chemse/bjaa081.
Chia KX, Polakhare S, Bruno SD. Possible affective cognitive cerebellar syndrome in a young patient with COVID-19 CNS vasculopathy and stroke. BMJ Case Rep. 2020;13(10):e237926. https://doi.org/10.1136/bcr-2020-237926.
Werner J, Reichen I, Huber M, Abela IA, Weller M, Jelcic I. Subacute cerebellar ataxia following respiratory symptoms of COVID-19: a case report. BMC Infect Dis. 2021;21:1–7. https://doi.org/10.5167/uzh-206294.
Shaikh AG, Manto M, Mitoma H. 2 years into the pandemic: what did we learn about the COVID-19 and cerebellum? Cerebellum. 2022;21(1):19–22. https://doi.org/10.1007/s12311-021-01351-7.
Bougakov D, Podell K, Goldberg E. Multiple neuroinvasive pathways in COVID-19. Mol Neurobiol. 2021;58(2):564–75. https://doi.org/10.1007/s12035-020-02152-5.
Chen B, Chen C, Zheng J, Li R, Xu J. Insights into neuroimaging findings of patients with coronavirus disease 2019 presenting with neurological manifestations. Front Neurol. 2020;11:593520. https://doi.org/10.3389/fneur.2020.593520.
Egbert AR, Cankurtaran S, Karpiak S. Brain abnormalities in COVID-19 acute/subacute phase: a rapid systematic review. Brain Behav Immun. 2020;89:543–54. https://doi.org/10.1016/j.bbi.2020.07.014.
Ntaios G, Michel P, Georgiopoulos G, Guo Y, Li W, Xiong J, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke: the global COVID-19 stroke registry. Stroke. 2020;51(9):e254–e8. https://doi.org/10.1161/STROKEAHA.120.031208.
Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020;194:105921. https://doi.org/10.1016/j.clineuro.2020.105921.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–44. https://doi.org/10.1093/eurheartj/ehaa623.
Walker KA, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, et al. Midlife systemic inflammatory markers are associated with late-life brain volume: the ARIC study. Neurology. 2017;89(22):2262–70. https://doi.org/10.1212/WNL.0000000000004688.
Walker KA, Windham BG, Power MC, Hoogeveen RC, Folsom AR, Ballantyne CM, et al. The association of mid-to late-life systemic inflammation with white matter structure in older adults: the atherosclerosis risk in communities study. Neurobiol Aging. 2018;68:26–33. https://doi.org/10.1016/j.neurobiolaging.2018.03.031.
Baig AM. Neurological manifestations in COVID-19 caused by SARS-CoV‑2. CNS Neurosci Ther. 2020;26(5):499. https://doi.org/10.1111/cns.13372.
Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073–85. https://doi.org/10.1002/acn3.51350.
World Health Organisation. Post COVID-19 condition: WHO supports standardization of clinical data collection and reporting. 2021. https://www.who.int/news/item/12-08-2021-post-covid-19-condition-who-supports-standardization-of-clinical-data-collection-and-reporting. Accessed 16 Jan 2023.
Global Health 50/50, The African Population and Health Research Center, International Center for Research on Women. The sex, gender and COVID-19 project. 2022. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/. Accessed 16 Jan 2023.
Liu X, Yan W, Lu T, Han Y, Lu L. Longitudinal abnormalities in brain structure in COVID-19 patients. Neurosci Bull. 2022; https://doi.org/10.1007/s12264-022-00913-x.
Shanley JE, Valenciano AF, Timmons G, Miner AE, Kakarla V, Rempe T, et al. Longitudinal evaluation of neurologic-post acute sequelae SARS-CoV‑2 infection symptoms. Ann Clin Transl Neurol. 2022;9(7):995–1010. https://doi.org/10.1002/acn3.51578.
Shehab M, Abualigah L, Shambour Q, Abu-Hashem MA, Shambour MKY, Alsalibi AI, et al. Machine learning in medical applications: a review of state-of-the-art methods. Comput Biol Med. 2022;145:105458. https://doi.org/10.1016/j.compbiomed.2022.105458.
Singh NM, Harrod JB, Subramanian S, Robinson M, Chang K, Cetin-Karayumak S, et al. How machine learning is powering neuroimaging to improve brain health. Neuroinformatics. 2022; https://doi.org/10.1007/s12021-022-09572-9.
Kang J, Chen T, Luo H, Luo Y, Du G, Jiming-Yang M. Machine learning predictive model for severe COVID-19. Infect Genet Evol. 2021;90:104737. https://doi.org/10.1016/j.meegid.2021.104737.
Khair AM, Nikam R, Husain S, Ortiz M, Kaur G. Para and post-COVID-19 CNS acute demyelinating disorders in children: a case series on expanding the spectrum of clinical and radiological characteristics. Cureus. 2022;14(3):1–12. https://doi.org/10.7759/cureus.23405.
Avila M, Tan YY, Hernandez R, Zuberi H, Rivera VM. Multiple sclerosis and neuromyelitis optica spectrum disorder: onset following acute COVID-19 infection, a case series. Neurol Ther. 2023;12(1):319–27. https://doi.org/10.1007/s40120-022-00418-9.
Applewhite BP, Buch K, Yoon BC, et al. Lung apical findings in coronavirus disease (COVID-19) infection on neck and cervical spine CT. Emerg Radiol. 2020;27(6):731–5. https://doi.org/10.1007/s10140-020-01822-0.
Abrams RMC, Safavi F, Tuhrim S, Navis A, Steinberger J, Shin SC. MRI negative myelopathy post mild SARS-CoV‑2 infection: vasculopathy or inflammatory myelitis? J Neurovirol. 2021;27(4):650–5. https://doi.org/10.1007/s13365-021-00986-w.
Mahammedi A, Saba L, Vagal A, et al. Imaging of neurologic disease in hospitalized patients with COVID-19: an Italian multicenter retrospective observational study. Radiology. 2020;297(2):E270–E3. https://doi.org/10.1148/RADIOL.2020201933.
Mehan WA, Yoon BC, Lang M, Li MD, Rincon S, Buch K. Paraspinal myositis in patients with COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(10):1949–52. https://doi.org/10.3174/ajnr.A6711.
Garg RK, Paliwal VK, Gupta A. Spinal cord involvement in COVID-19: a review. J Spinal Cord Med. 2023;46(3):390–404. https://doi.org/10.1080/10790268.2021.1888022.
Okrzeja J, Garkowski A, Kubas B, Moniuszko-Malinowska A. Imaging and neuropathological findings in patients with post COVID-19 neurological syndrome—a review. Front Neurol. 2023; https://doi.org/10.3389/fneur.2023.1136348.
Dressing A, Bormann T, Blazhenets G, Schroeter N, Walter LI, Thurow J, August D, Hilger H, Stete K, Gerstacker K, Arndt S, Rau A, Urbach H, Rieg S, Wagner D, Weiller C, Meyer PT, Hosp JA. Neuropsychologic profiles and cerebral glucose metabolism in neurocognitive long COVID syndrome. J Nucl Med. 2022;63(7):1058–63. https://doi.org/10.2967/jnumed.121.262677.
Rau A, Schroeter N, Blazhenets G, Dressing A, Walter LI, Kellner E, Bormann T, Mast H, Wagner D, Urbach H, Weiller C, Meyer PT, Reisert M, Hosp JA. Widespread white matter oedema in subacute COVID-19 patients with neurological symptoms. Brain. 2022;145(9):3203–13. https://doi.org/10.1093/brain/awac045.
Faro SH, Manmatharayan A, Leiby B, Jain N, Mohamed FB, Talekar KS, Doshi A, Jambor I, Chang S, Finkelstein M, Kremer S, Lersy F, Lindgren B, Figueiredo NM, Gerevini S, Napolitano A, Jain R, Dogra S, Pillai JJ, Ryan D, Jager R, Carletti F, Mian A, Kaliev A, Anand P, Takahashi C, Murat A, Colen R, Mansueto G, Pizzini F. Neuroimaging findings in 4342 hospitalized COVID-19 subjects: a multicenter report from the United States and Europe. J Neuroimaging. 2023; https://doi.org/10.1111/jon.13140.
Funding
This project is supported by Bournemouth University (2021/22) Quality-Related (QR) Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
C. Kiyak, O.A. Ijezie, J.A. Ackah, M. Armstrong, J. Cowen, D. Cetinkaya, H. Burianová and T.N. Akudjedu declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Ceyda Kiyak and Ogochukwu Ann Ijezie are first co-authors.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiyak, C., Ijezie, O.A., Ackah, J.A. et al. Topographical Distribution of Neuroanatomical Abnormalities Following COVID-19 Invasion. Clin Neuroradiol 34, 13–31 (2024). https://doi.org/10.1007/s00062-023-01344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-023-01344-5