Abstract
Aim
To evaluate neuropathology and neuroradiology in the diagnosis and clinical outcome of a retrospective cohort of thalamic gliomas.
Methods
Neuropathological and neuroradiological review was undertaken in 25 cases of radiologically suspected thalamic glioma (excluding childhood pilocytic astrocytoma) over an 8 year period (2004–2012) at Frenchay Hospital and compared to the clinical outcome.
Results
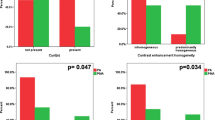
In 12/25 (48 %) there was a difference in neuropathological and suspected neuroradiological grading of the lesion of one or more grades. In 5/12 (42 %) cases, the neuroradiology was lower grade than the pathology. In 4/5 (80 %) of these cases, we identified a minimally enhancing subtype where the neuroradiology was predicted to be of lower grade than neuropathology. In 4/12, (33 %) the suspected neuroradiology grade was higher than the final pathology. In 3/4, (75 %) of these cases the suspected neuroradiology grade was higher than the neuropathology possibly because of unusual differentiation within the thalamic glioma (central neurocytoma, anaplastic oligoastrocytoma, and diffuse astrocytoma with pilocytic features). In 3/12 (25 %) the biopsy was non-diagnostic. Neuropathology was a better predictor of clinical outcome than neuroradiology. 9/10 (90 %) WHO Grade 4 gliomas and 8/9 (88 %) Grade 3 gliomas on neuropathology were dead between 3−7 years after diagnosis. 3/3 (100 %) Grade 2 gliomas on neuropathology were alive 3–7 years after diagnosis. 2/3 (67 %) of the non-diagnostic cases were alive 3–7 years after biopsy. In 1/3 (33 %) of the non-diagnostic cases the outcome was unknown.
Conclusions
Diagnosis of primary thalamic glioma is challenging. We have identified that in the thalamus, a pattern of diffuse infiltration with minimal enhancement on imaging may often represent high-grade glioma. Neuropathology is overall the best predictor of clinical outcome.





Similar content being viewed by others
References
Beks JWF, Bouma GJ, Journée HL. Tumours of the thalamic region. Acta Neurochir. 1987;85:125–7.
Nishio S, Morioka T, Suzuki S, Takeshita I, Fukui M. Thalamic gliomas: a clinicopathologic analysis of 20 cases with reference to patient age. Acta Neurochir. 1997;139:336–42.
Franzini A, Leocata F, Cajola L, Servello D, Allegranza A, Broggi G. Low-grade glial tumours in basal ganglia and thalamus: natural history and biological reappraisal. Neurosurgery. 1994;35(5):817–21.
Pathy S, Jayalakshmi S, Chander S, Singh R, Julka PK, Rath GK. Prognostic factors influencing the outcome of thalamic glioma. Neurol India. 2002;50(1):37–40.
Krouwer HGJ, Prados M. Infiltrative astrocytomas of the thalamus. J Neurosurg. 1995;82:548–57.
McKissock W, Paine KWE. Primary tumours of the thalamus. Brain. 1958;81(1):41–63.
Burtscher IM, Skagerberg G, Geijer B, Englund E, Stahlberg F, Holtas S. Proton MR spectroscopy and pre-operative diagnostic accuracy: an evaluation of intracranial mass lesions characterized by stereotactic biopsy findings. AJNR Am J Neuroradiol. 2000;21:84–93.
Cheung YK. Central neurocytoma occurring in the thalamus: CT and MRI findings. Australas Radiol.1996;40(2):182–4.
Croteau D, Scarpace L, Hearshen D, Gutierrez J, Fisher JL, Rock JP, et al. Correlation between magnetic resonance spectroscopy imaging and image-guided biopsies: semiquantitative and qualitative histopathological analyses of patients with untreated glioma. Neurosurgery. 2001;49(4):823–9.
McGirt M, Villavicencio AT, Bulsara KR, Friedman AH. MRI-guided stereotactic biopsy in the diagnosis of glioma: comparison of biopsy and surgical resection specimen. Surg Neurol. 2003;59:277–82.
Swanson KR, Alvord EC, Murray JD. Virtual brain tumours (gliomas) enhance the reality of medical imaging and highlight inadequacies of current therapy. Br J Cancer. 2002;86:14–8.
Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol. 2003;24(10):1989–98.
Kondziolka D, Lunsford LD, Martinez AJ. Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg. 1993;79:533–6.
Price SJ, Burnet NG, Donovan T, Green HA, Peña A, Antoun NM, et al. Diffusion tensor imaging of brain tumours at 3T: a potential tool for assessing white matter tract invasion? Clin Radiol. 2003;58(6):455–62.
Lu S, Ahn D, Johnson G, Cha S. Peritumoral diffusion tensor imaging of high-grade gliomas and metastatic brain tumours. AJNR Am J Neuroradiol. 2003;24:937–41.
Di Costanzo A, Pollice S, Trojsi F, Giannatempo GM, Popolizio T, Canalis L, et al. Role of perfusion-weighted imaging at 3 Tesla in the assessment of malignancy of cerebral gliomas. Radiol med. 2008;113(1):134–43.
Lupo JM, Cha S, Chang SM, Nelson SJ. Dynamic susceptibility-weighted perfusion imaging of high-grade gliomas: characterization of spatial heterogeneity. AJNR Am J Neuroradiol. 2005; 26:1446–54.
Choi C, Ganji SK, DeBerardinis RJ, Hatanpaa KJ, Rakheja D, Kovacs Z, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18(4):624–9.
Burnet NG, Lynch AG, Jefferies SJ, Price SJ, Jones PH, Antoun NM, et al. High-grade glioma: imaging combined with pathological grade defines management and predicts prognosis. Radiother Oncol. 2007;85(3):371–8.
Conflict of interest
The authors declare that there is no actual or potential conflicts of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurian, K.M., Zhang, Y., Haynes, H.R. et al. Diagnostic Challenges of Primary Thalamic Gliomas—Identification of a Minimally Enhancing Neuroradiological Subtype with Aggressive Neuropathology and Poor Clinical Outcome. Clin Neuroradiol 24, 231–238 (2014). https://doi.org/10.1007/s00062-013-0225-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-013-0225-y