Abstract
Infections of clinical methicillin-resistant Staphylococcus aureus (MRSA) is a very tough public health problem and a challenge of new drug development. Nearly 90 Diels-Alder adducts (DAAs) have so far been isolated from Morus plants, but only a few of them have been evaluated for their anti-MRSA activities. To study the antibacterial compounds of DAAs from the root barks’ section of Morus alba L. and their synergism with antibacterial agents against clinical MRSA strains, bioassay-guided phytochemical methods were used to screen the active components. Minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) were assayed through broth serial microdilution. The synergism were evaluated by checker board microdilution and dynamic time-kill experiments. Three DAAs (multicaulisin (1), sanggenon G (2) and albanin G (3)) were isolated and identified from M. alba root barks. They were determined with potent effect against MRSA isolates with MICs/MBCs at 2–8/16–128 mg/L. They also showed synergy with conventional antibacterial agents, especially the aminoglycosides, with fractional inhibitory concentration indices (FICIs) ranged from 0.19 to 0.50 and the dose reduction indices (DRIs) ranged from 16 to 2. The MRSA resistance to the antibiotics could be reversed by compounds 1–3. The dose-dependent bactericidal synergism against MRSA was observed as well. The study released for the first time the anti-MRSA synergism of DAAs from M. alba root barks with antibacterial agents and the reversal of MRSA resistance to aminoglycosides. The results may be valuable for further development of new antibacterial drugs and synergists against MRSA infections.
Similar content being viewed by others
Introduction
Staphylococcus aureus is a clinical prevalent pathogen causing diseases from mild skin and soft tissue to life-threatening infections, especially the infections with methicillin-resistant S. aureus (MRSA) by higher rates of in-hospital complication and mortality, and greater health care costs (Guillamet et al. 2018). Currently, MRSA can be resistant to most conventional antibacterial agents (Mercer et al. 2017), worse yet the occurrence of vancomycin-resistant S. aureus (VRSA) (Okada et al. 2018). A serious lack of new anti-MRSA drugs has promoted the search for novel chemical entities from natural sources. For a number of years, we are working on finding the novel phytochemicals from medicinal plants based on their uses in traditional Chinese medicine (TCM), especially those which can potentiate the anti-MRSA effect and even reverse the MRSA resistance to conventional antibacterial agents (Zuo et al. 2018).
Morus alba L. (Moraceae) is a typical plant of genus Morus, the perennial deciduous shrub or tree with a height of 3–15 m. It is distributed throughout Asia, Africa, Europe, and South and North America. The root bark of M. alba is a TCM known as Sang-Bai-Pi (SBP) and has been documented in the Chinese Pharmacopoeia. SBP has been widely used for asthma, edema, and other diseases since 500 BC. Over 110 compounds have been found from SBP, including flavonoid derivatives of Diels-Alder adducts (DAAs), 2-arylbenzofurans and stilbenes. The pharmacological effects of these compounds, such as anti-microbic, anti-inflammatory, anti-tumor and anti-oxidative activities, were also reported (Zuo et al. 2018; Wei et al. 2016).
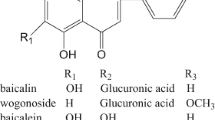
It was claimed that nearly 90 DAAs have been found from SBP and other Morus plants, however, only a few of them have been subjected to the evaluation of anti-MRSA properties (Ross et al. 2008; Ku et al. 2010; Farooq et al. 2015). As part of our continuing studies on the new anti-MRSA phytochemicals from SBP (Zuo et al. 2018), we herein report the anti-MRSA activity and potentiation effect of three known DAAs from SBP, i.e., multicaulisin (Mu, 1; Ferrari et al. 2000), sanggenon G (SG, 2; Fukai et al. 1983) and albanin G (kuwanon H, moracenin A) (AG, 3; Cui 2008; Nomura et al. 2009), mainly dealing with their synergism on aminoglycosides against clinical MRSA isolates by classic checker board microdilution (CBMD) and time-killing curve (T-KC) methods.
Materials and methods
General phytochemical procedures
1H- and 13C-NMR spectra (TMS as internal standard) were determined on a Bruker AM-400 NMR spectrometer. MS spectra were recorded on a VG Auto Spec-3000 mass spectrometer. Column chromatography was accomplished on si-gel (200–300 mesh and 300–400 mesh, Qingdao Marine Chemical Co., Ltd., Qingdao, China) and Sephadex LH-20 (40–70 µm; Amersham Pharmacia Biotech AB, Uppsala, Sweden). Fractions and isolated compounds were monitored by TLC (GF254, Qingdao Marine Chemical Co., Ltd., Qingdao, China) through visualizing by 5% FeCl3 (in 80% ethanol) and 10% H2SO4 (in ethanol) reagents, respectively.
Plant materials
The root barks of M. alba (SBP; voucher specimen KUN 0515476, Herbarium of KIB) were purchased from Yunnan Lv Sheng Pharmaceutical Co., Ltd, Kunming, China in Aug. 2010. The samples were washed thoroughly, air-dried at room temperature, coarsely powdered by a grinder (Suining General Machinery Factory, Sichuan, China) and stored in sterile airtight containers.
Antibacterial agents and disks
The four antibacterial agents including three aminoglycosides and a floroquinolone were purchased from the manufacturers in China, i.e., amikacin (Ak) (Jiangsu Wuzhong Pharmaceutical Group Co., Ltd.); etimicin (Em) (Wuxi Jiming Kexin Shanhe Pharmaceutical Co., Ltd., Wuxi China); gentamicin (Gm) and levofloxacin (Le) (Yangtze River Pharmaceutical Group Co., Ltd., Taizhou China). Vancomycin (Va) was bought from Eli Lilly Japan K. K., Seishin Laboratories. Antibiotic impregnated disks of cefoxitin (0.03 mg) and others were supplied by Tiantan biological products Co., Ltd., Beijing, China. The three DAAs 1–3 were prepared from SBP (purity ≥95% by 13C NMR spectral analysis; Supplementary material, S1).
Bacterial strains and media
Ten clinical MRSA strains of SCCmec III type were obtained and characterized using cefoxitin disk and multiplex PCR tests as previously reported (Zuo et al. 2018). MSSA (ATCC 25923) was used as the control strain. Mueller-Hinton (M-H) agar and broth (Tianhe Microbial Agents Co., Hangzhou, China) were used as bacterial culture media. All susceptibility assays including time-kill curves were carried out in M-H broth. The bacteria were grown at 35 °C for 24 h and examined in daylight. Colony counts in M-H agar plates were manually made under microscope.
Preparation of DAAs 1–3 from SBP
Preparation of extracts
The pulverized plant sample (5000 g) was immersed and extracted with 80% ethanol at room temperature for three times (7, 3 and 3 days, respectively). The filtered liquid sections were combined and evaporated the solvent at 40 °C under reduced pressure to get the crude extracts (635 g, 12.7%). The crude extracts were suspended in deionized water (1000 mL) and further sequentially extracted using ethyl acetate and n-butanol (Zuo et al. 2018). The resulted sub-sections 240 and 40 g, together with 355 g from water phase were stored at −20 °C until tested for the inhibition against MRSA.
Bioassay-guided isolation of the DAAs
The ethyl acetate extracts (240 g) which showed the most effective in the three sub-sections on MRSA were chromatographed through si-gel column (200–300 mesh), gradient elution with petroleum ether-ethyl acetate (10:1–1:1) to give 20 fractions (Sfr-1–20); further screening of the active fractions and repeated column chromatography of Sfr-12 with si-gel (300–400 mesh, chloroform-methanol (30:1 and 15:1, respectively)) to furnish compound 3. Further repeated chromatography of Sfr-13 with si-gel (300–400 mesh, chloroform-methanol (30:1 and 10:1, respectively) and Sephadex LH-20 (methanol) afforded compounds 1 and 2, respectively. Compounds 1–3 were subjected to spectral analyses and compared the data with those of previously reported (Ferrari et al. 2000; Fukai et al. 1983; Cui 2008).
Multicaulisin
Mu (1) was isolated as reddish brown powder (methanol); ESI-MS: m/z (%) 715 (100) [M + Na]+; 1H-NMR (400 MHz in CD3OD) δ: 7.34 (1H, d, J = 8.8, H-6′), 7.15 (1H, b d, H-27), 6.81 (1H, br s, H-3), 6.15 (1H, br d, H-24), 6.49 (1H, d, J = 2.3 Hz, H-3′), 6.46 (1H, dd, J = 8.8, 2.3 Hz, H-5′), 6.42 (1H, br s, H-8), 6.13 (1H, d, J = 2.2 Hz, H-30), 6.08 (1H, dd, J = 2.2, 8.2 Hz, H-32), 6.06 (1H, dd, J = 2.4, 8.8 Hz, H-24), 5.93 (1H, d, J = 2.4 Hz, H-24), 5.29 (1H, br s, H-10), 5.22 (1H, brt, H-14), 4.92 (1H, br m, H-20), 4.34 (1H, br d, H-9), 3.70 (1H, br m, H-19), 1.45–1.64 (2H, br m, H-12, 13, 18), 1.35 (3H, s, H-16), 1.28 (3H,s,H-17). 13C-NMR (100 MHz in CD3OD) δ: 210.3 (C-21), 184.0 (C-4), 166.0 (C-23), 165.8 (C-25), 162.6 (C-7,4′), 161.9 (C-2,5), 161.2 (C-2′), 157.9 (C-31), 156.9 (C-29), 134.5 (C-11), 132.9 (C-33), 132.7 (C-6′), 132.3 (C-27), 125.0 (C-10), 124.8 (C-14), 123.1 (C-28), 116.8 (C-22), 113.8 (C-6), 108.7 (C-1′), 108.3 (C-5′), 108.1 (C-3), 107.9 (C-32), 105.7 (C-26), 103.8 (C-3′), 103.6 (C-24), 103.0 (C-30), 98.6 (C-8), 48.7 (C-20), 39.2 (C-9), 26.1 (C-12, 18), 24.9 (C-19), 23.6 (C-13), 23.3 (C-16), 17.8 (C-17). These data were in agreement with that of multicaulisin as the previous report (Ferrari et al. 2000).
Sanggenon G
SG (2) was isolated as brown powder (methanol)), ESI-MS: m/z (%) 717 (100) [M + Na]+; 1H-NMR (400 MHz in CD3OD) δ: 7.61 (1H, br d, J = 8, H-27), 7.17 (1H, d, J = 8, H-6′), 6.82 (1H, d, J = 8, H-33), 6.31 (1H, d, J = 2, H-3′), 6.29 (1H, dd, J = 2, 8 Hz, H-5′), 6.12 (1H, d, J = 2.5, H-30), 6.07 (1H, dd, J = 2.5, 8 Hz, H-26), 6.06 (lH, dd, J = 2.5, 8 Hz, H-32), 5.97 (1H, d, J = 2.5, H-24), 5.70 (1H, s, H-8), 5.54 (1H, br d, J = 13, H-2), 5.20 (1H, m, H-14), 3.02 (1H, dd, J = 13, 16 Hz, trans-H-3), 2.50–2.68 (1H, m, cis-H-3), 2.05–2.16 (4H, m, Hx2-12, 13), 1.63, 1.68 (each 3H, s, H-15). 13C-NMR (100 MHz in CD3OD) δ: 210.8 (C-21), 198.4 (C-4), 166.2 (C-7), 166.0 (C-5), 163.4 (C-23, 25), 162.6 (C-8a), 159.7 (C-4′), 157.3 (C-29, 31), 156.8 (C-2′), 132.2 (C-33), 129.1 (C-15), 128.9 (C-27), 125.7 (C-14), 118.0 (C-28), 116.2 (C-22), 110.5 (C-1′), 108.5 (C-5′), 107.9 (C-6), 107.7 (C-32), 107.4 (C-26), 103.8 (C-24), l03.4 (C-4a), 103.0 (C-30), 102.7 (C-3′), 96.2 (C-8), 75.6 (C-2), 43.2 (C-3), 38.7 (C-12), 27.5(C-13), 26.1 (C-16), 18.0 (C-17). These data were in agreement with that of sanggenon G as the previous report (Fukai et al. 1983).
Albanin G
AG (3) was isolated as reddish brown powder (methanol)), ESI-MS: m/z (%) 783 (100) [M + Na]+; 1H-NMR (400 MHz in CD3OD) δ (ppm): 1.47 (3H, s, H-13), 1.50 (3H, s, H-7″), 1.54 (3H, s, H-25″), 1.60 (3H, s, H-12), 1.65 (3H, s, H-24″), 1.94 (1H, m, H-6″), 2.00 (1H, overlapped, H-6″), 3.11 (4H, d, J = 7.2 Hz, H-9, 21″), 3.22 (1H, m, H-5″), 4.42 (1H, br d, J-9.6 Hz, H-3″), 4.65 (1H, br s, H-4″), 5.05 (1H, t, J = 7.2 Hz, H-22″), 5.16 (1H, t, J = 9.6 Hz, H-10), 5.20 (1H, br s, H-2″), 5.98 (1H, d, J = 8.4 Hz, H-13″), 5.99 (1H, s, H-6), 6.05 (1H, br d, J-8.4 Hz, H-19″), 6.20 (1H, br s, H-17″), 6.55 (1H, br d, J = 8.4 Hz, H-5′), 6.65 (1H, br s, H-3′), 6.85 (1H, d, J = 8.4 Hz, H-20″), 7.26 (1H, d, J = 8.4 Hz, H-6′), 7.82 (1H, d, J = 8.4 Hz, H-14″); 13C-NMR (100 MHz in CD3OD) δ (ppm): 209.5 (C-8″), 183.9 (C-4), 163.6 (C-10″), 163.0 (C-8ª,12″), 161.7 (C-4′), 161.3 (C-2), 161.0 (C-7), 157.7 (C-5, 18″), 156.8 (C-2″, 8″), 134.4 (C-1″), 132.7 (C-23″), 132.1 (C-11, 20″), 131.6 (C-6′), 130.7 (C-14″), 124.6 (C-2″), 123.6 (C-15″, 22″), 122.9 (C-10), 121.5 (C-3), 115.7 (C-11″), 115.3 (C-9″), 113.7 (C-1′), 108.7 (C-5′, 19″), 107.8 (C-8), 107.3 (C-13″), 105.6 (C-4a), 103.6 (C-3′, 17″), 98.5 (C-6), 47.5 (C-4″), 38.1 (C-3″, 5″, 6″), 25.9 (C-12, 24″), 24.7 (C-9), 23.1 (C-7″), 22.3 (C-21″), 17.8 (C-25″), 17.7 (C-13). These data were in agreement with that of albanin G as the previous reports (Cui 2008).
Susceptibility testing
The minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) of DAAs 1–3 were measured by standard method of CLSI as previous report (Zuo et al. 2018). Briefly, test samples of DAAs 1–3 were dissolved in 5% aqueous DMSO to obtain a stock solution of 1024 mg/L. Serial broth microdilution of the samples with M-H broth from 1024–2 mg/L were performed in the 96-well flat-bottomed microplates. The well containing no sample served as a negative control. Each well contained final bacterial inoculums of 5 × 105 CFU/ml. The plates were incubated at 35 °C for 24 h. MIC was defined as the lowest concentration at which no turbidity was observed after incubation. Aliquots (0.02 mL) removed from wells with no bacterial growth were streaked onto M-H agar plates and incubated under the same conditions. MBC was defined as the lowest concentration at which the growth of colonies counted less than five after incubation. They were determined in duplicate. Vancomycin was used as the positive control drug.
Synergy testing
The bacteriostatic synergism of a combination was assessed as previously reported (Zuo et al. 2018), using CBMD method by criteria of the fractional inhibitory concentration indices (FICIs) as synergy, FICI ≤ 0.5; indifferent, 0.5 < FICI ≤ 4; antagonism, FICI > 4. The degree of potentiation was determined according to a dose reduction index (DRI) which was calculated as DRI = MICalone/MICcombined. The corresponding bactericidal synergism was evaluated by the T-KC experiments through the increase of killing in log10CFU/mL at the 24 h incubation (ΔLC24), i.e.: ΔLC24 ≥ 2 log10CFU/mL, synergy; ΔLC24 = 1–2 log10CFU/mL, additive; ΔLC24 = ±1 log10CFU/mL, indifferent; ΔLC24 > −1 log10CFU/mL, antagonism.
Cell cytotoxicity
The cytotoxicity of compounds (2 and 3) were tested against human cell lines of liver HL-7702 and lung cancer A549 (Procell, Wuhan, China) using the MTS assay as the previous report (Zuo et al. 2018).
Results
The identified active DAAs 1–3 from SBP extracts
Bioassay-guided fractionation of the SBP extracts resulted in the identification of three anti-MRSA compounds DAAs 1–3 (Fig. 1). Their spectral data were in agreement with those reported in the literature (Ferrari et al. 2000; Fukai et al. 1983; Cui 2008; S1).
Potent activity and synergism of DAAs 1–3 on MRSA
Tables 1 and 2 show the activity of DAAs 1–3 with MIC90 ≤8 and 2 mg/L (n = 10) used alone and in combination with the antibacterial agents, respectively. Generally, DAAs 1–3 each used alone was determined with activity more potent than any of the combined aminoglycosides (Ak, Em, and Gm) and a floroquinolone (Le), with the exception of Va. There are four to nine MRSA strains (n = 10) showing synergy in the six combinations of DAAs 1–3 with the three aminoglycosides Ak, Em and Gm, with FICI50 ranged from 0.375 to 0.563 and DRI50 from 8 to 4 (Table 2). None of the combinations showed antagonism.
Reversal of MRSA resistance to aminoglycosides by DAAs 1–3
Table 3 shows the reversal effect of DAAs 1–3 on MRSA resistance to the tested aminoglycosides. According to the CLSI interpretive criteria (i.e., MICs ≤ 16 mg/L) (CLSI 2012), all combinations of Ak with DAAs 1–3 against the ten MRSA isolates showed susceptible (S) because the combined MICs were 2–16 mg/L, which revealed that the resistance of all MRSA strains (n = 10) to Ak was reversed. Similarly, Mu caused the resistance of five MRSA isolates against Gm to be reversed, but AG showed no resistance reversal on Le against MRSA (Table 3). Em also showed MICs ≤1 mg/L in the combinations with Mu and SG against nine MRSA isolates, the same potency against the MSSA (Tables 1–3).
Dose dependent killing effects on MRSA by aminoglycosides in combination with DAAs 1 and 2
The potential bactericidal synergism by DAAs 1–3 were further evaluated through the classical dynamic T-KC experiment using MR09, a MRSA strain listed in Table 1. As the results displayed in Table 4 and Fig. 2, there are three combinations (i.e., SG + Ak, AG + Ak and AG + Le) exhibiting synergy of bactericidal effect at 1 × MIC of the interacted concentrations revealed by the criterion of increase in killing at 24 h (ΔLC24 ≥ 2 log10 CFU/mL) (CLSI 2012), as the ΔLC24 were calculated as 2.59, 4.11, and 2.11 log10 CFU/mL, respectively (ΔLC24 = LC24Md − LC24co; Table 4).
Time-kill curves of the synergistic effect of the combination of multicaulisin (Mu), sanggenon G (SG) and albanin G (AG) at 1 × MIC (alone concentration) a–ga, and (1–4) × MIC (alone concentrations) of Mu and SG Hb, respectively, against MA07, a clinical methicillin-resistant Staphylococcus aureus strains of SCCmec III type. Results are mean of triplicate samples. aΔLC24 = LC24Md − LC24co (e.g., in f AG + Ak showed synergy as ΔLC24 = 4.11, corresponding to the value in Table 4). bThe trend of the fold lines in H indicates that as the concentration of Mu and SG increases, the CFU/mL value of LC24 gradually approaches zero, i.e., the time-kill effects following the order of Mu < SG < 2Mu < 4Mu < 2SG < 4SG
The dose-dependent synergistic effects were demonstrated by (1–1/4) × MIC of the interacted aminoglycosides with 1 × MIC of DAAs 1 and 2, respectively. The results are shown in Table 4. In the combinations of Ak with SG, decreasing concentrations of Ak to 1/2 MIC also displayed equally significant synergy with 1 × MIC of SG with ΔLC24 = 2.59 log10 CFU/mL, but only the additive effect (ΔLC24 = 1.89 log10 CFU/mL) at 1/4 MIC of Ak was observed (Table 4). Beside 1 × MIC, Em at 1/2 and 1/4 MIC all showed additive effects with 1 × MIC of SG as well. Meanwhile, Em with the concentrations of (1–1/4) × MIC showed interaction with Mu as additive, additive and indifferent effects, respectively (Table 4). Nevertheless, the differences of ΔLC24 were yet statistically significant with the changes of concentration for the antibacterial agents. For the concentrations of DAAs other than 1 × MIC used alone, the increases of killing effects dose-dependently against MRSA at the concentrations from 1 to 4 × MICs were significantly observed, with even the LC24 of SG under 2 and 4 ×MICs both reduced to zero (Fig. 2h).
In vitro cell cytotoxicity of sanggenon G and albanin G
The cytotoxicity of Mu and SG were assayed with IC50 values as 3.6, 4.4 mg/L and 3.4, 3.0 mg/L on HL-7702 and A549 cell lines, respectively.
Discussion
MRSA was first reported by Jevons in 1961 (Jevons 1961), and has since been evolved as a seriously global healthcare problem. There is the emergence of MRSA with reduced susceptibility to vancomycin and even VRSA occurred (Okada et al. 2018). It was estimated that antimicrobial resistance (AMR) would cause 10 million deaths a year by 2050 (De Kraker et al. 2016). For this reason, the World Health Organization (WHO) has already listed anti-MRSA new drug development as high priority (WHO 2017). Unfortunately, however, the lack of innovation in antibiotics development and the “paradox” in the practical superiority validation for the proposed drug over the existing drugs may make AMR problem (including MRSA) more tricky, and thus there remains concerning the crisis that we may return to the pre-antibiotic era (Fears and Ter Meulen 2014; Rex et al. 2017).
Therefore, the rational treatment regimens of current antibacterial agents, and strategies of making the existent antibacterial agents to be sustainably effective and reversing the resistant agents to return to be susceptible to AMR pathogens become vital important. Alternatively, synergism between plant natural products and antibiotics has been proposed as approaching a new generation of phytopharmaceuticals (Hemaiswarya et al. 2008; Wagner and Ulrich-Merzenich 2009). Zacchino et al. (2017) also reviewed the plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Hence, results of the present report added new phytochemical potentials of the approach.
In this report, we demonstrated that the DAAs 1–3 from SBP showed potent anti-MRSA activities used alone. More significantly, we also firstly found the synergism of them in combination with current antibacterial agents, especially aminoglycosides, against MRSA with potent effects in fighting against the problematic AMR pathogen. Other antibiotics, such as penicillins and cephalosporins, showed no synergism with these compounds in the preliminary experiment. Apart from anti-MRSA effects of the DAAs herein, AG also showed other pharmacological effects, including anti-inflammatory, anti-hypertensive, HIV, and tyrosinase inhibitory effects (Nomura et al. 2009; Wei et al. 2016), and used as a weight loss ingredient (Yimam et al. 2017). SG was reported inhibition against the neuraminidases (Grienke et al. 2016), GABA (A) receptor (Çiçek 2018) and an apoptosis protein (XIAP) which was viewed as an attractive target for anti-cancer therapy (Seiter et al. 2014). AG and SG were also previously reported with potent anti-MRSA activity (i.e., MICs of 0.3–3 mg/L) (Ku et al. 2010). The MIC differences from our results might be attributed to the assay with varied MRSA strains.
Although previous reports have also involved the antimicrobial and cytotoxic activity of other SBP ingredients (Wei et al. 2016), including other Morus DAAs against MRSA, i.e., artonin I (Farooq et al. 2015), and Sorocenols G and H (Ross et al. 2008). However, the anti-MRSA and cytotoxic activity of Mu and SG, especially the synergism of DAAs 1–3 has so far been reported herein for the first time. It is noted that a natural DAA from Morus spp. is formed by a Diels-Alder reaction between α, β-olefinic moiety of a chalcone and an isoprene moiety. Generally, they are phenolic phytochemicals. Therefore, they are likely to have similar effects on MRSA which warrant further investigations in the future.
DAAs 1–3 contain the same moieties of tetrahydroxyflavone (1 and 3) or tetrahydroxyflavanone (2), including the same double-resorcinol moieties in the structures (Fig. 1). It is thus not surprising that the (MICs) MSSA and the 50 and 90% of the combined (MICs)MRSA and DRIs in the combinations were not different significantly (Table 2). Moreover, DAAs in the combinations might have played a leading role in reducing the MIC values of combinatory usage as well (Tables 2–4, Fig. 2). It could be seen that the DRI value of an antibacterial agent in a combination is usually greater than that of a DAA, which well illustrates DAAs as the potentiators of the tested antibacterial agents (Table 2).
Bacterial pathogens have evolved AMR to current drugs via varied mechanisms including the receptor (or active site) modification and enzymatic modification /degradation of the drugs, and reducing accumulation of the drugs within the bacterial cells (decreased membrane permeability and active efflux). Therefore, the anti-AMR action of a phytochemical used alone or in combination with an antibacterial drug would be also to counteract one or more of these resistant mechanisms through (1) synergistic multi-target effects; (2) enzymatic inhibition or increasing bioavailability; (3) combined interactions on bacterial resistance mechanisms and (4) elimination or neutralization of adverse effects by the added agents, so that altogether a better effectiveness than without these additions or manipulations can be achieved (Wagner and Ulrich-Merzenich 2009; Hemaiswarya et al. 2008). It was revealed that artonin I, one of the DAA-type phytochemical with similar activity as DAAs 1–3, could depolarize bacterial cell membrane and inhibit efflux mechanism and induce cellular destruction through cell membrane damage (Farooq et al. 2015), which implies that DAAs 1–3 may also exhibit the similar mechanisms and remains to be clarified.
Although DAAs 1–3 are extraordinarily active alone against MRSA, their potent cytotoxicity does not seem to be beneficial for anti-MRSA treatment. However, the combined use of DAAs 1–3 with the antibacterial agents could largely reduce their respective MIC50 to below the respective IC50 (Table 2), result in anti-MRSA potency approaching to vancomycin (Tables 1 and 2). Therefore, both the toxicity of DAAs and the agents could be reduced. There could also be possibly beneficial for the DAAs as potential antitumor agents, i.e., achieving multi-target chemotherapy of tumor patients with bacterial infection at the same time (Seiter et al. 2014), considering systematic studies have shown that the Gram-positive organisms are the leading cause of invasive bacterial disease in patients with cancer (Holland et al. 2014).
The broad-spectrum antibiotics of aminoglycoside suffer from issues of nephrotoxicity and ototoxicity. We also noted that Table 2C entitled “Zone Diameter and MIC Breakpoints for Staphylococcus spp.” in the 28th CLSI Performance Standards only retained the gentamicin breakpoint (Table 3a) (CLSI 2018). Even so, in the last decade, the field of aminoglycoside antibiotics has experienced a renewed interest among the scientific community and efforts were made to counter the resistance and toxicity associated with these drugs. The reversal of MRSA resistances herein might add this interest in the renaissance to aminoglycoside (Fair and Tor 2014; Chandrika and Garneautsodikova 2016; Krause et al. 2016). In cases where aminoglycosides are required to be used, it is vital for controlling infection with a smaller dosage, highlighting the importance of which DAAs are used in combination with such antibacterial agents. In the TCM treatment, a crude drug is usually not used individually. Multiple crude drugs employed in a TCM decoction may produce synergistic effects of increased potency and decreased toxicity, similar to the synergism in the present research, i.e., the adverse effects of either the DAAs or the antibiotics would be reduced when they are used in combination attributable to the reduced dosage.
Therefore, the future work is necessary to perform in-depth studies on the in vivo efficacy. It is warrant to enrich more amounts of DAAs 1–3 and to isolate additional DAAs to explore the potential mechanisms and other pharmacological aspects, including evaluation of more antibacterial agents showing synergy on the compounds or their synthetic derivatives both with effectiveness but less toxicity.
Conclusion
Our study details DAAs, i.e., multicaulisin (1), sanggenon G (2) and albanin G (3) as the potent in vitro anti-MRSA constitution in the root barks of M. alba (SBP) extract, justifying the effects of DAAs 1–3 on synergism with conventional antibacterial agents. The data were the first time presented so far for the synergistic anti-MRSA effects of the novel chemical entities of DAAs 1–3 on three conventional antibacterial agents of aminoglycosides amikacin, etimicin, gentamicin, and a floroquinolone (levofloxacin), and the reversal of MRSA resistance to the aminoglycosides, especially amikacin. The results could be beneficial for developing new anti-infectious drugs or synergists against MRSA and warrant further studies on mechanisms of action and in vivo pharmacological efficacy.
Abbreviations
- AG:
-
albanin G
- Ak:
-
amikacin
- AMR:
-
antimicrobial resistance
- CFU:
-
colony forming unit
- CBMD:
-
checker board microdilution
- CLSI:
-
Clinical and Laboratory Standards Institute
- DAA:
-
Diels-Alder adduct
- DRI:
-
dose reduction index
- Em:
-
etimicin
- Gm:
-
gentamicin
- FICI:
-
fractional inhibitory concentration index
- I:
-
intermediate
- Le:
-
levofloxacin
- MBC:
-
minimal bactericidal concentration
- M-H:
-
Mueller-Hinton medium
- MIC:
-
minimal inhibitory concentration
- MSSA:
-
methicillin-susceptible Staphylococcus aureus
- MRSA:
-
methicillin-resistant Staphylococcus aureus
- Mu:
-
multicaulisin
- NMR:
-
nuclear magnetic resonance
- R:
-
resistant
- S:
-
susceptible
- SBP:
-
Sang-Bai-Pi (root barks of Morus alba L. (Moraceae))
- SG:
-
sanggenon G
- T-KC:
-
time-killing curve
- Va:
-
vancomycin
References
Chandrika NT, Garneautsodikova S (2016) A review of patents (2011-2015) towards combating resistance to and toxicity of aminoglycosides. Medchemcomm 7(1):50–68
Çiçek SS (2018) Structure-dependent activity of natural GABA (A) receptor modulators. Molecules 23(7):1512
Clinical Laboratory Standards Institute (2012) Table 2CZone Diameter and MIC Interpretive Standards for Staphylococcus sppIn Performance Standards for Antimicrobial Susceptibility Testing Twenty-Second Informational Supplement Approved Standard M100-S22Wayne PA CLSI
Clinical and Laboratory Standards Institute (CLSI) (2018) Performance Standards for Antimicrobial Susceptibility Testing 28th ed CLSI supplement M100Wayne PA CLSI
Cui XQ (2008) Studies on the chemical constituents and bioactivities of Morus yunnanensis Koidz and Faeces bombycis Chinese Academy of Medical Sciences, Peking Union Medical College (dissertation)
Fair RJ, Tor Y (2014) Antibiotics and bacterial resistance in the 21st century. Perspec Med Chem 6(6):25–64
Farooq S, Wahab AT, Fozing CDA, Rahman AU, Choudhary MI (2015) Artonin I inhibits multidrug resistance in Staphylococcus aureus and potentiates the action of inactive antibiotics in vitro. J Appl Microb 117(4):996–1011
Fears R, Ter Meulen V (2014) What do we need to do to tackle antimicrobial resistance? Lancet Glob Health 2(1):e11–e12
Ferrari F, Monache FD, Suarez AI, Compagnone RS (2000) Multicaulisin a new Diels-Alder type adduct from Morus multicaulis. Fitoterapia 71(2):213–215
Fukai T, Hano Y, Fujimoto T, Nomura T (1983) Structure of sanggenon G a new Diels-Alder adduct from the Chinese crude drug “Sang-Bai-Pi” (Morus root barks). Heterocycles 20(4):611–615
Grienke U, Richter M, Walther E, Hoffmann A, Kirchmair J, Makarov V, Nietzsche S, Schmidtke M, Rollinger JM (2016) Discovery of prenylated flavonoids with dual activity against influenza virus and Streptococcus pneumonia. Sci Rep 6:27156
Guillamet MCV, Vazquez R, Deaton B, Shroba J, Vazquez L, Mercier RC (2018) Host-pathogen-treatment triad host factors matter most in methicillin-resistant Staphylococcus aureus bacteremia outcomes. Antimicrob Agents Chemother 62(2):1–10
Hemaiswarya S, Kruthiventi AK, Doble M (2008) Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 15:639–652
Holland T, Fowler VG, Shelburne SA (2014) Invasive Gram-positive bacterial infection in cancer patients. Clin Infect Dis 59(suppl 5):S331–S334
Jevons MP (1961) “Celbenin”-resistant staphylococci. Br Med J 1:124–125
Kraker MEAD, Stewardson AJ, Harbarth S (2016) Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med 13(11):e1002184
Krause KM, Serio AW, Kane TR, Connolly LE (2016) Aminoglycosides an overview. Cold Spring Harb Perspec Med 6:a027029
Ku YL, Liang ST, Lin YH, Chen LH, Kuo YY (2010) Anti-bacterial use of extract from Morus australis poir. and compound kuwanon H 2011 US8071142B2
Mercer DK, Katvars LK, Hewitt F, Smith DW, Robertson J, O’Neil DA (2017) NP108 an Antimicrobial polymer with activity against methicillin-and mupirocin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 61(9):1–12
Nomura T, Hano Y, Fukai T (2009) Chemistry and biosynthesis of isoprenylated flavonoids from Japanese mulberry tree. Proc Jpn Acad SerB 85(9):391–408
Okada N, Fujita T, Kanamori J, Sato A, Horikiri Y, Sato T, Fujiwara H, Daiko H (2018) A case report of postoperative VRSA enteritis effective management of rifampicin for vancomycin resistant Staphylococcus aureus enteritis after esophagectomy and colon reconstruction. Int J Surg Case Rep 52:75–78
Release News (2017) WHO publishes list of bacteria for which new antibiotics are urgently needed. Neurosciences 38 (4):444–445
Rex JH, Talbot GH, Goldberger MJ, Eisenstein BI, Echols RM, Tomayko JF, Dudley MN, Dane A (2017) Progress in the fight against multidrug-resistant bacteria 2005–2016 modern noninferiority trial designs enable antibiotic development in advance of epidemic bacterial resistance. Clin Infect Dis 65(1):141–146
Ross SA, Rodríguezguzmán R, Radwan MM, Jacob M, Ding Y, Li XC, Ferreira D, Manly SP (2008) Sorocenols G and H anti-MRSA oxygen heterocyclic Diels-Alder-type adducts from Sorocea muriculata roots. J Nat Prod 71(10):1764–1767
Seiter MA, Salcher S, Rupp M, Hagenbuchner J, Kiechlkohlendorfer U, Mortier J, Wolber G, Judith M, Rollinger JM, Obexer P, Ausserlechner MJ (2014) Discovery of sanggenon G as a natural cell-permeable small-molecular weight inhibitor of X-linked inhibitor of apoptosis protein (XIAP). FEBS Open Bio 4:659–671
Wagner H, Ulrich-Merzenich G (2009) Synergy research approaching a new generation of phytopharmaceuticals. Phytomedicine 16:97–110
Wei H, Zhu JJ, Liu XQ, Feng WH, Wang ZM, Yan LH (2016) Review of bioactive compounds from root barks of Morus plants (Sang-Bai-Pi) and their pharmacological effects. Cogent Chem 2:1212320
Yimam M, Jiao P, Hong M, Brownell L, Lee YC, Hyun EJ, Kim HJ, Nam JB, Kim MR, Jia Q (2017) UP601 a standardized botanical composition composed of Morus alba Yerba mate and Magnolia officinalis for weight loss. BMC Complement Alter Med 17(1):114
Zacchino SA, Butassi E, Liberto MD, Raimondi M, Sortino M (2017) Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 37:27–48
Zuo GY, Yang CX, Han J, Li YQ, Wang GC (2018) Synergism of prenylflavonoids from Morus alba root bark against clinical MRSA isolates. Phytomedicine 39:93–99
Acknowledgements
The research was supported from No 81173504 (NSFC, China) and 2008PY001 (Yunnan Province, China). The authors wish to thank Kunming Institute of Botany (KIB, CAS) for spectral analysis.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zuo, GY., Yang, CX., Ruan, ZJ. et al. Potent anti-MRSA activity and synergism with aminoglycosides by flavonoid derivatives from the root barks of Morus alba, a traditional Chinese medicine. Med Chem Res 28, 1547–1556 (2019). https://doi.org/10.1007/s00044-019-02393-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-019-02393-7