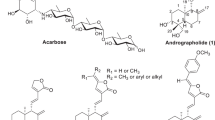
Abstract
A series of ring-A fused heterocycles of lupane, oleanane, ursane and dammarane triterpenoids were synthesized and evaluated for their inhibitory activity against α-glucosidase. An influence of the different types of triterpenoids with indole and pyrazine cycles on the activity was revealed. Among them, 2,3-indolo-lup-20(29)-en-28-oic acid with an IC50 of 1.8 µM was the most active compound being 221-fold more active than the market drug acarbose. In the most cases, the replacement of the indole by the pyrazine fragment provided the decreasing of activity (except dammarane type pyrazine derivative).
Graphical Abstract






Similar content being viewed by others
References
Abdullah NH, Salim F, Ahmad R (2016) Chemical сonstituents of malaysian U. cordata var. ferruginea and their in vitro α-glucosidase inhibitory activities. Molecules 21:525–536
Bednarczyk-Cwynar B (2014) An overview on the chemistry and biochemistry of triterpenoids. Mini Rev Org Chem 11:251–252
Chianese G, Yerbanga SR, Lucantoni L, Habluetzel A, Basilico N, Taramelli D, Fattorusso E, Taglialatela-Scafati Q (2010) Antiplasmodial triterpenoids from the fruits of neem, Azadirachta indica. J Nat Prod 73:1448–1452
Dang Z, Ho Ph, Zhu L, Qian K, Lee K-H, Huang L, Chen Ch-H (2013) New betulinic acid derivatives for bevirimat-resistant human immunodeficiency. J Med Chem 56:2029–2037
Finlay JH, Honda T, Gribble GW (2002) Synthesis of novel [3,2-b]indole fused oleanolic acids as potential inhibitors of cell proliferation. ARKIVOC 12:38–46
Flekhter OB, Nigmatullina LR, Baltina LA, Karachurina LT, Galin FZ, Zarudii FS, Tolstikov GA, Boreko EI, Pavlova NI, Nikolaeva SN, Savinova OV (2002) Synthesis of betulinic acid from betulin extract and study of the antiviral and antiulcer activity of some related terpenoids. Pharm Chem J 36:484–487
Flekhter OB, Boreko EI, Nigmatullina LR, Pavlova NI, Medvedeva NI, Nicolaeva SN, Tret’yakova EV, Savinova OV, Baltina LA, Karachurina LT, Galin FZ, Zarudii FS, Tolstikov GA (2004) Synthesis and pharmacological activity of acylated betulonic acid oxides and 28-Oxo-allobetulone. Pharm Chem J 38:148–152
Fontanay S, Grare M, Mayer J, Finance C, Duval RE (2008) Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J Ethnopharmacol 120:272–276
Genet C, Strehle A, Schmidt C, Boudjelal G, Lobstein A, Schoonjans K, Souchet M, Auwerx J, Saladin R, Wagner A (2010) Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: potential impact in diabetes. J Med Chem 53:178–190
Giniyatullina GV, Smirnova IE, Kazakova OB, Yavorskaya NP, Golubeva IS, Zhukova OS, Pugacheva RB, Aprysko GN, Poroikov VV (2015) Synthesis and anticancer activity of aminopropoxytriterpenoids. Med Chem Res 24:3423–3436
Hida T, Fukui Y, Kawata K, Kabaki M, Masui T, Fumoto M, Nogusa H (2010) Practical application of oxidation using a novel Na2WO4-H2O2 system under neutral conditions for scale-up manufacturing of 12α-hydroxy-3-oxooleanano-28,13-lactone: key intermediate of endothelin A receptor antagonist S-0139. Org Process Res Dev 14:289–294
Ikeda Y, Murakami A, Ohigashi H (2008) Ursolic acid: an anti- and pro-inflammatory triterpenoid. Mol Nutr Food Res 52:26–42
Jeppesen PB, Gregersen S, Rolfsen SE, Jepsen M, Colombo M, Agger A, Xiao J, Kruhøffer M, Orntoft T, Hermansen K (2003) Antihyperglycemic and blood pressure-reducing effects of stevioside in the diabetic Goto-Kakizaki rat. Metabolism 52:372–378
Kazakova OB, Giniyatullina GV, Yamansarov EY, Tolstikov GA (2010) Betulin and ursolic acid synthetic derivatives as inhibitors of Papilloma virus. Bioorg Med Chem Lett 20:4088–4090
Khusnutdinova EF, Smirnova IE, Giniyatullina GV, Medvedeva NI, Yamansarov EY, Kazakov DV, Kazakova OB, Linh PT, Viet Q, Huong DT (2016) Inhibition of alpha-glucosidase by synthetic derivatives of lupane, oleanane, ursane and dammarane triterpenoids. Nat Prod Comm 11:33–35
Kim YM, Wang MH, Rhee HI (2004) A novel α-glucosidase inhibitor from pine bark. Carbohydr Res 339:715–717
Kumar V, Rani N, Aggarwal P, Sanna VK, Singh AT, Jaggi M, Joshi N, Sharma PK, Irchhaiya R, Burman AC (2008) Synthesis and cytotoxic activity of heterocyclic ring-substituted betulinic acid derivatives. Bioorg Med Chem Lett 18:5058–5062
Liu J, Chen D, Liu P, He M, Li J, Li J, Hu L (2014) Discovery, synthesis, and structure-activity relationships of 20(S)-protopanaxadiol (PPD) derivatives as a novel class of AMPKα2β1γ1 activators. Eur J Med Chem 79:340–349
López D, Cherigo L, Spadafora C, Loza-Mejía MA, Martínez-Luis S (2015) Phytochemical composition, antiparasitic and α-glucosidase inhibition activities from Pelliciera rhizophorae. Chem Cent J 9:53
Nie W, Luo J-G, Wang X-B, Yin H, Sun H-B, Yao H-Q, Kong L-Y (2011) Synthesis of new α-glucosidase inhibitors based on oleanolic acid incorporating cinnamic amides. Chem Pharm Bull 59:1051–1056
Niwa T, Doi U, Osawa T (2003) Inhibitory activity of corn-derived bisamide compounds against alpha – glucosidase. J Agric Food Chem 51:90–94
Paduch R, Kandefer-Szerszen M, Trytek M, Fiedurek J (2007) Terpenes: substances useful in human healthcare. Arch Immunol Ther Exp (Warsz) 46:2652–2661
Qi L-W, Liu E-H, Chu C, Peng Y-B, Cai H-X, Li P (2010) Anti-diabetic agents from natural products - an update from 2004 to 2009. Curr Top Med Chem 10:434–457
Sheng H, Sun H (2011) Synthesis, biology and clinical significance of pentacyclic triterpenes: a multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat Prod Rep 28:543–593
Silva FSG, Oliveira PJ, Duarte MF (2016) Oleanolic, ursolic, and betulinic acids as food supplements or pharmaceutical agents for type 2 diabetes: promise or illusion? J Agric Food Chem 64:2991–3008
Smirnova IE, Houng DoTT, Kazakova OB, Tolstikov GA, Kukovinets OS, Lobov AN, Suponitsky KY (2012) Ozonolisis of dipterocarpol and its derivatives. Russ J Org Chem 48:1370–1376
Smirnova IE, Kazakova OB, Huong Do TT, Minnibaeva EM, Lobov AN, Suponitsky KY (2014) One-pot synthesis of hollongdione from dipterocarpol. Nat Prod Commun 9:1417–1420
Smirnova IE, Kazakova OB, Do Quoc Viet, Nguyen Thi T, Pham Thyu L, Do Thi Thu H (2015) Synthesis and evaluation of 29-norcycloartane triterpenoids as α-glucosidase inhibitors. Med Chem Res 24:2177–2182
Sommerwerk S, Csuk R (2014) Convenient and chromatography-free partial syntheses of maslinic acid and augustic acid. Tetrahedron Lett 55:5156–5158
Tolstikov GA, Kim H-O, Gorayev MI (1967) Synthesis of triterpenoid indols. Zh Obshch Khim 37:1960
Urban M, Sarek J, Kvasnica M, Tislerova I, Hajduch M (2007) Triterpenoid pyrazines and benzopyrazines with cytotoxic activity. J Nat Prod 70:526–532
Vlk M, Micolova P, Urban M, Kvasnica M, Saman D, Sarek J (2016) 15N-labelled pyrazines of triterpenic acid. J Radioanal Nucl Chem 308:733–739
Wang YY, Yang YX, Zhe H, He ZX, Zhou SF (2014) Bardoxolone methyl (CDDO-Me) as a therapeutic agent: an update on its pharmacokinetic and pharmacodynamic properties. Drug Des Dev Ther 2014:2075–2088
Wu PP, Zhang K, Lu YJ, He P, Zhao SQ (2014) In vitro and in vivo evaluation of the antidiabetic activity of ursolic acid derivatives. Eur J Med Chem 80:502–508
Yasue M, Sakakibara J, Kaiya J (1974) Syntheses of nitrogen-containing triterpenes. II. Ring-A fused triterpenes containing nitrogen. Yakugaku Zasshi 94:461–465
Acknowledgements
The analyses were performed on the equipment of the Center of joint usage “Khimiya” of Ufa Institute of Chemistry, Russian Academy of Sciences. The work was partially financially supported by the Russian Foundation for Basic Research (project no. 10-03-90303). EFK gratefully acknowledges the President of Russian Federation for a young scientist stipendium 2016-2018 (SP-1507.2016.4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Khusnutdinova, E.F., Smirnova, I.E., Kazakova, O.B. et al. Synthesis and evaluation of 2,3-indolotriterpenoids as new α-glucosidase inhibitors. Med Chem Res 26, 2737–2742 (2017). https://doi.org/10.1007/s00044-017-1972-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-017-1972-0