Abstract
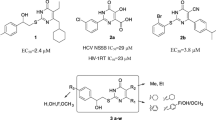
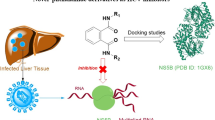
In this work, a series of oxime ether phenylpropanoid derivatives were synthesized. Their anti-hepatitis B virus (HBV) activity in HepG 2.2.15 cells was determined, and anti-cancer potential against three human cancer cell lines was evaluated. All the synthesized derivatives showed great efficiency against HBV. Compound 4d demonstrated the most effective anti-HBV activity, performing strong potent inhibitory not only on the secretion of HBsAg (IC50 = 50.45 μM, SI = 9.18) and HBeAg (IC50 = 50.11 μM, SI = 9.24), but also on the HBV DNA replication (IC50 = 51.80 μM, SI = 8.94). Besides, the synthetic compounds also displayed obvious anti-cancer activity. Moreover, the docking study of all synthesized compounds inside the related protein active site was conducted to explore the molecular interactions and a molecular target for activity using a MOE-docking technique. This study identified a new class of potent anti-HBV and anti-cancer agents.







Similar content being viewed by others
References
Babu KR, Rao VK, Kumar YN, Polireddy K, Subbaiah KV, Bhaskar M, Lokanatha V, Raju CN (2012) Identification of substituted [3, 2-a] pyrimidines as selective antiviral agents: molecular modeling study. Antivir Res 95:118–127
Balzarini J, Orzeszko-Krzesinska B, Maurin JK, Orzeszko A (2009) Synthesis and anti-HIV studies of 2- and 3-adamantyl-substitutedthiazolidin-4-ones. Eur J Med Chem 44:303–311
Brittelli David R (1981) Phosphite-mediated in situ carboxyvinylation: a new general acrylic acid synthesis. J Org Chem 46:2514–2520
Chen DF, Zhang SX, Xie L et al (1997) Anti-AIDS agents—XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorgan Med Chem 5:1715–1723
Chen H, Ma YB, Huang XY, Geng CA, Zhao Y, Wang LJ, Guo RH, Liang WJ, Zhang XM, Chen JJ (2014) Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg Med Chem Lett 24:2353–2359
Du NN, Li X, Wang YP, Liu F, Liu YX, Li CX, Peng ZG, Gao LM, Jiang JD, Song DQ (2011) Synthesis, structure-activity relationship and biological evaluation of novel N-substituted matrinic acid derivatives as host heat-stress cognate 70 (Hsc70) down-regulators. Bioorg Med Chem Lett 21:4732–4735
Ferrari M, Fornasiero MC, Isetta AM (1990) MTT colorimetric assay for testing macrophage cytotoxic activity in vitro. J Immunol Methods 131:165–172
Gao LM, Han YX, Wang YP, Li YH, Shan YQ, Li X, Peng ZG, Bi CW, Zhang T, Du NN, Jiang JD, Song DQ (2011) Design and synthesis of oxymatrine analogues overcoming drug resistance in hepatitis B virus through targeting host heat stress cognate 70. J Med Chem 54:869–876
Han YQ, Huang ZM, Yang XB, Liu HZ, Wu GX (2008) In vivo and in vitro anti-hepatitis B virus activity of total phenolics from Oenanthe javanica. J Ethnopharmacol 18:148–153
Karakurt A, Mehmet AA, Burcu S, Çalıs Ü, Dalkara S (2012) Synthesis of some novel 1-(2-naphthyl)-2-(imidazol-1-yl)ethanone oxime ester derivatives and evaluation of their anticonvulsant activity. Eur J Med Chem 57:275–282
Kim SN, Lee JY, Kim HJ, Shin CG, Parka H, Lee YS (2000) Synthesis and HIV-1 integrase inhibitory activities of caffeoylglucosides. Bioorg Med Chem Lett 10:1879–1882
Lavanchy D (2004) Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 11:97–107
Liu JX, Kenneth Y, Chen ECR (2011) Structural insights into the binding of hepatitis B virus core peptide to HLA-A2 alleles: towards designing better vaccines. Eur J Immunol 41:2097–2106
Liu S, Wei WX, Shi KC, Cao X, Zhou M, Liu ZP (2014a) In vitro and in vivo anti-hepatitis B virus activities of the lignan niranthin isolated from Phyllanthus niruri L. J Ethnopharmacol 155:1061–1067
Liu S, Wei WX, Li YB, Lin X, Shi KC, Cao X, Zhou M (2014b) In vitro and in vivo anti-hepatitis B virus activities of the lignan nirtetralin B isolated from Phyllanthus niruri L. J Ethnopharmacol 157:62–68
Liu S, Wei WX, Li YB, Liu X, Cao XJ, Lei KC, Zhou M (2015) Design, synthesis, biological evaluation and molecular docking studies of phenylpropanoid derivatives as potent anti-hepatitis B virus agents. Eur J Med Chem 95:473–482
Manat M, Patil B (2007) Evaluation of antiinflammatory activity of methanol extract of Phyllanthus amarus in experimental animal models. Indian J Pharm Sci 69:33
Ovenden SP, Yu J, San WS et al (2004) Globoidnan A: a lignan from Eucalyptus globoidea inhibits HIV integrase. Phytochemistry 65:3255–3259
Panda P, Appalashetti M, Natarajan M, Mary CP, Venkatraman SS, Judeh ZMA (2012a) Synthesis and antiproliferative activity of helonioside A,3′,4′,6′-tri-O-feruloylsucrose, lapathoside C and their analogs. Eur J Med Chem 58:418–430
Panda P, Appalashetti M, Natarajan M, Chan-Park MB, Venkatraman SS, Judeh ZMA (2012b) Synthesis and antitumor activity of lapathoside D and its analogs. Eur J Med Chem 53:1–12
Parvathaneni M, Battu GR, Gray AI et al (2014) Investigation of anticancer potential of hypophyllanthin and phyllanthin against breast cancer by in vitro and in vivo methods. Asian Pac J Trop Dis 4:S71–S76
Peterson JR, Russell ME, Surjasasmita IB (1988) Synthesis and experimental ionization energies of certain (E)-3-arylpropenoic acids and their methyl esters. J Chem Eng Data 33:534–537
Quan VV, Trenerry C, Rochfort S, Wadeson J, Leyton C, Hughes AB (2013) Synthesis and anti-inflammatory activity of aromatic glucosinolates. Bioorgan Med Chem 21:5945–5954
Saleem M, Kim HJ, Ali MS et al (2005) An update on bioactive plant lignans. Nat Prod Rep 22:696–716
Salum ML, Robles CJ, Erra-Balsells R (2010) Photoisomerization of ionic liquid ammonium cinnamates: one-pot synthesis-isolation of Z-cinnamic acids. Org Lett 12:4808–4811
Salway AH (1909) Synthesis of substances allied to cotarnine. J Chem Soc Trans 95:1204–1220
Santos FD, Abreu P, Castro HC, Paixao ICPP, Cirne-Santos CC, Giongo V, Barbosa JE, Simonetti BR, Garrido V, Bou-Habib DC, Silva DD, Batalha PN, Temerozo JR, Souza TM, Nogueira CM, Cunha AC, Rodrigues CR, Ferreira VF, Souza MCBV (2009) Synthesis, antiviral activity and molecular modeling of oxoquinoline derivatives. Bioorgan Med Chem 17:5476–5481
Terent’ev AO, Krylov IB, Ogibin YN, Nikishin GI (2006) Chlorination of oximes with aqueous H2O2/HCl system: facile synthesis of gem-chloronitroso- and gem-chloronitroalkanes, gem-chloronitroso- and gem-chloronitrocycloalkanes. Synthesis 22:3819–3824
Viegas-Junior C, Danuello A, Bolzani VS, Barreir EJ, Fraga CAM (2007) Molecular hybridization: a useful tool in the design of new drug prototypes. Curr Med Chem 14:1829–1852
Wang LJ, Geng CA, Ma YB, Huang XY, Luo J, Chen H, Guo RH, Zhang XM, Chen JJ (2012) Synthesis, structure–activity relationships and biological evaluation of caudatin derivatives as novel anti-hepatitis B virus agents. Bioorg Med Chem Lett 20:2877–2888
Wei WX, Li XR, Wang KW, Zheng ZW, Zhou M (2012) Lignans with anti-hepatitis B virus activities from phyllanthus niruri L. Phytother Res 26:964–968
Wu ZR, Zheng LF, Li Y, Su F, Yue XX, Tang W, Ma XY, Nie JY, Li HY (2012) Synthesis and structure–activity relationships and effects of phenylpropanoid amides of octopamine and dopamine on tyrosinase inhibition and antioxidation. Food Chem 134:1128–1131
Yamashita K, Nohara Y, Katayama K et al (1992) Sesame seed lignans and gamma-tocopherol act synergistically to produce vitamin E activity in rats. J Nutr 122:2440–2446
Zou HB, Wu H, Zhang XN, Zhao Y, Joachim S, Lou YJ, Yu YP (2010) Synthesis, biological evaluation, and structure-activity relationship study of novel cytotoxic aza-caffeic acid derivatives. Bioorgan Med Chem 18:6351–6359
Acknowledgments
This work was financially supported by the national natural science foundation of China, natural science foundation of Guangxi province, China, and science research and technology development foundation of Guangxi province, China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, S., Li, Y., Wei, W. et al. Synthesis and biological evaluation of phenylpropanoid derivatives. Med Chem Res 25, 1074–1086 (2016). https://doi.org/10.1007/s00044-016-1554-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1554-6