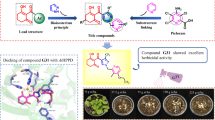
Abstract
Aconitine compounds are found in Aconitum Ouwu head, Chuan Wu, Aconitum, and other Ranunculaceae aconitum plants, which are not only active ingredient but also toxic component. In present study, 20 antitumor target proteins of different types were selected from Protein Data Bank (http://www.rcsb.org), for the purpose of finding potential antitumor targets of aconitum alkaloids, top ranked proteins were screened by molecular docking method, using the docking module in Sybyl-X 1.1 and Molecular Operating Environment (MOE) 2008, and screening result was verified by protein–ligand interaction fingerprint (PLIF) in MOE. Mesaconitine showed a C-shaped conformation when docking into heat-shock protein 90 (HSP90), which was similar with ANSA ring of geldanamycin. And the PLIF indicated that they shared many common amino acid residues interacted with HSP90; equally, Yunaconitine was found having similar conformation with the inhibitor of poly ADP-ribose polymerase-1 (PARP-1).








Similar content being viewed by others
References
Feng PW, Qi CF (1981) Alkaloids from roots of Aconitum crassicaule. Planta Med 42:375–379
Gangloff AR, Brown J, Jong R, Dougan DR, Grimshaw CE, Hixon M, Vu P (2013) Discovery of novel benzo [b][1,4] oxazin-3(4H)-ones as poly (ADP-ribose) polymerase inhibitors. Bioorg Med Chem Lett 23:4501–4505
Gao LM, Mao XF, Wei XM, Zheng S (1999) Diterpenoid alkaloids of pharmacological effects and structure–activity relationship profiles. Chin J Northwest Norm Univ Nat Sci 39:98–102
Hazawa M, Wada K, Takahashi K, Mori T, Kawahara N, Kashiwakura I (2009) Suppressive effects of novel derivatives prepared from Aconitum alkaloids on tumor growth. Investig New Drugs 27:111–119
Hu H, Rao Z, Xu J, Zhu Q, Altenbach H-J, Chen H, Zhou D, Xiao Y, Ke X, Guo H (2012) 16-Morpholino quaternary ammonium steroidal derivatives as neuromuscular blocking agents: synthesis, biological evaluation and in silico probe of ligand-receptor interaction. Eur J Med Chem 56:332–347
Huang YR (1991) Aconitum cancer studies. Fujian Trad Chin Med 22:54–56
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. Cancer J Clin 60:277–300
Kitchen DB, Decornez H, Furr JR, Bajorath J (2004) Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov 3:935–949
Lewell XQ, Smith R (1997) Drug-motif-based diverse monomer selection: method and application in combinatorial chemistry. J Mol Graph Model 15:43–48
Li ZJ, Wang L, Peng J, Yan XL, Meng FH (2013) Study on the quantitative structure–toxicity relationships of aconitine compounds basing on PCA-ANN method. Med Chem Res 10:4964–4969
Meng S, Shan GZ, Li ZR (2011) Recent progress in the study of Hsp90 inhibitors. Chin J Antibiot 36:241–248
Mohammed KAH, Atef AAH, Nawal AEK, Nadia MM, Alessio I, Claudiu TS (2007) Design, synthesis, and docking studies of new 1,3,4-thiadiazole-2-thione derivatives with carbonic anhydrase inhibitory activity. Bioorgan Med Chem 15:6975–6984
MOE Molecular Operating Environment (2008) version 2008. Chemical Computing Group Inc., Montreal, QC, Canada
Nishanov AA, Sultankhodzhaev MN, Yunusov MS, Kondrat’ev VG (1991) Alkaloids of Aconitum rubicundum. Chem Nat Compd 27:349–352
Pockley AG (2003) Heat shock proteins as regulators of the immune response. Lancet 362:469–476
Prema LM, Manali J, James MB (2012) Pharmacophore-based virtual screening to aid in the identification of unknown protein function. Chem Biol Drug Des 80:828–842
Ren XW, Yi PL, Shao MW (2003) Comparative evaluation of 11 scoring functions for molecular docking. J Med Chem 46:2287–2303
Roe SM, Prodromou C, O’Brien R, Ladbury JE, Piper PW, Pearl LH (1999) Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 42:260–266
Swarup NT (1961) Isolation and detection of aconitine in toxicological analysis. Fresen J Anal Chem 180:36–37
SYBYL, Tripos Inc. http://www.tripos.com/
Tan L, Lounkine E, Bajorath J (2008) Similarity searching using fingerprints of molecular fragments involved in protein–ligand interactions. J Chem Inf Model 48:2308–2312
Tulp M, Bohlin L (2002) Functional versus chemical diversity: is biodiversity important for drug discovery? Trends Pharmacol Sci 23:225–231
Wang YX, Jia K, Zhang DS (1989) Aconitine on immune function in rats. Trad Chin Med Info 9:40–41
Zhapova T, Modonova LD, Semenov AA (1985) Mesaconitine and hypaconitine from Aconitum czekanovskyi. Chem Nat Compd 21:678–679
Acknowledgments
This work was supported by Chinese national science foundation with Grant Nos. 81274182, 81303315, 81573687.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Liang, Jw., Zhang, Tj., Li, Zj. et al. Predicting potential antitumor targets of Aconitum alkaloids by molecular docking and protein–ligand interaction fingerprint. Med Chem Res 25, 1115–1124 (2016). https://doi.org/10.1007/s00044-016-1553-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1553-7