Abstract
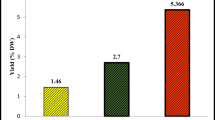
The essential oils obtained by the hydrodistillation from the fresh flowers, leaves, stems, and roots of Ferula communis L., growing in Tunisia were analyzed by GC and GC/MS. Thirty-two components were identified in the oil of flowers with camphor (18.3 %), α-pinene (15.3 %), and β-eudesmol (9.3 %) as the main constituents. Twenty-nine compounds were identified in the oil of stems with β-eudesmol (28.1 %), δ-eudesmol (11.1 %), and α-eudesmol (9.6 %) as the main compounds. Twenty compounds were characterized in the oil of roots with dillapiole (7.9 %), guaiol (7.3 %), and spathulenol (6.8 %). In the oil of leaves, α-eudesmol (25.2 %), β-eudesmol (20.7 %), δ-eudesmol (10.1 %), and caryophyllene oxide (7.2 %) were found as the main constituents. This study was undertaken to evaluate the antioxidant activity using DPPH (2,2′-diphenyl-1-picrylhydrazyl), ABTS (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid), reducing power, and catalase activity. We tested also the antibacterial, cytotoxic, and cholinesterase inhibition properties of the essential oil of different organs of F. communis. The essential oil of the stems showed the highest antioxidant activity (IC50 = 0.03 ± 0.001 mg mL−1), in DPPH assay and the important result of catalase (303.03 µmol H2O2 degraded/min/protein) of F. communis. The antibacterial activity of the oil was determined by micro-well dilution assay. The best results (MIC = 0.156 ± 0.02 mg mL−1) were exhibited by the essential oil of the leaves of F. Communis against Pseudomonas aeruginosa. Besides, the strongest cytotoxic activity against Hela cells was shown with essential oils’ leaves with an inhibition percentage of 79.05 % at the concentration of 500 µg mL−1. However, the best inhibition percentage of A 549 cells was detected for essential oils’ leaves with an inhibition percentage of 54.56 % at 250 µg mL−1. Our finding showed that the essential oil of the flowers was the most active, with 64.623 % of inhibition against butyrylcholinesterase at 10 mg mL−1 from the incubation time of 30 min.



Similar content being viewed by others
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometry. Allured Publishing, Carol Stream IL
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Al-Abrash ASA, Al-Quobaili FA, Al-Akhras GN (2000) Catalase evaluation in different human diseases associated with oxidative stress. Saudi Med J 21(9):826–830
Appendino G, Cravotto G, Sterner O, Ballero M (2001) Oxygenated sesquiterpenoids from a nonpoisonous Sardinian chemotype of giant fennel (Ferula communis). J Nat Prod 64:393–395
Aragno M, Tagliapietra S, Nano GM, Ugazia G (1988) Experimental studies on the toxicity of Ferula communis in the rat. Res Commun Chem Pathol Pharmacol 59:399–402
Asili J, Sahebkar A, Fazly Bazzaz BS, Sharifi S, Iranshahi M (2009) Identification of essential oil components of Ferula badrakema fruits by GC–MS and 13C-NMR methods and evaluation of its antimicrobial activity. J Essent Oil Bear Plants 12:7–15
Bellakhdar J (1978) Ferula communis L. In: Tech. Nord-Africaine (ed) Médecine Traditionnelle et Toxicologie Ouest Sahariennes. Tech. Nord-Africaine, Rabat, pp 287–288
Chalchat JC, Garry RP (1997) Correlation between chemical composition and antimicrobial activity. VI. Activity of some african essential oils. J Essent Oil Res 9:67–75
Davies NW (1990) Gas chromatographic retention indexes of monoterpenes and sesquiterpenes on methyl silicone and carbowax 20 M phases. J Chromatogr 503:1–24
Dehghan G, Solaimanian R, Shahverdi AR, Amin G, Mo Abdollahi, Shafiee A (2007) Chemical composition and antimicrobial activity of essential oil of Ferula szovitsiana DC. Flavour Fragr J 22:224–227
Del-Vechio-Vieira G, Sousa OV, Yamamoto CH, Kaplan MAC (2009) Chemical composition and antimicrobial activity of the essential oils of Ageratum fastigiatum (Asteraceae). Rec Nat Prod 3(1):52–57
Ellman GL, Courtney KD, Andres V, Featherstone M (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–90
Ferrari B, Tomi F, Casanova JJ (2005) Composition and chemical variability of Ferula communis essential oil from Corsica. Flavour Fragr J 20:180–185
Ghannadi A, Amree S (2002) Volatile oil constituents of Ferula gummosa Boiss. From Kashan, Iran. Essent Oil Res J 14:420–421
Ghannadi A, Sajjadi SE, Beigihasan A (2002) Composition of the essential oil of Ferula ovina (Boiss.) Boiss. From Iran. Daru 10:164–167
Goryaev MI, Sharipova FS, Tikhonova LK, Elchibekova LA (1968) Components of essential oils, XXXI. Essential oil of Ferula penninervis (Stalks). Russ J Appl Chem (Zhurnal Prikladnoi Khimii) 41:2745–2750
Halliwell B, Gutteridge JMC (1999) The chemistry of the free radicals and related reactive species. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine, 3rd edn. Oxford Science Publications, Oxford, pp 36–104
Hatano T, Kagawa H, Yasuhara T, Okuda T (1988) Two new flavonoids and other constituents in licorice root; their relative astringency and radical scavenging effects. J Chem Pharm Bull 36:2090–2097
Jabrane A, Ben Jannet H, Mighri Z, Mirjolet JF, Duchamp O, Harzallah-Skhiri F, Lacaille-Dubois MA (2010a) Two new sesquiterpene derivatives from the tunisian endemic Ferula tunetana Pom. J Chem Biodivers 7:392–399
Jabrane A, BenJannet H, Mastouri M, Mighri Z, Casanova J (2010b) Chemical composition and in vitro evaluation of antioxidant and antibacterial activities of the root oil of Ridolfia segetum (L.) Moris from Tunisia. J Nat Prod Res 24(6):491–499
Jennings W, Shibamoto T (1980) Qualitative analysis of favor and fragrance volatiles by glass capillary chromatography. Academic Press, New-York, p 472
Joshi SC, Verma AR, Mathela CS (2010) Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. J Chem Toxicol 48:37–40
Kapahi BK, Thappa RK, Aggarwal SG, Sarin YK (1985) Essential oil of Ferula jaesekheana Valtke. PAFAI J 7:23–24
Keskin M, Biçer O, Gül S, Can E (2004) A study on using Ferula communis (Chakshir) for oestrus synchronization in Shami (Damascus) goats under East-Mediterranean Condition of Turkey. In: Book of Abstract of the 55th Annual Meeting of the European Association for Animal Production. Bled, Slovenia, Theatre S3.6, p. 234
Kluge WH, Kluge HH, Bauer HI, Pietsch S, Anders J, Venbrocks RA (2001) Acetylcholinesterase assay for cerebrospinal fluid using bupivacaine to inhibit butyrylcholinesterase. BMC Biochem 2:1–8
Kose ED, Aktas O, Deniz IG, Sarikurkcu C (2010) Chemical composition, antimicrobial and antioxidant activity of essential oil of endemic Ferula lycia Boiss. J Med Plants Res 4:1698–1703
Maggi F, Cecchini C, Cresci A, Coman MM, Tirillini B, Sagratini G, Papa F (2009) Chemical composition and antimicrobial activity of the essential oil from Ferula glauca L. (F. communis L. subsp. glauca) growing in Marche (central Italy). Fitoterapia 80:68–72
Manolakou S, Tzakou O, Yannitsaros A (2013) Volatile constituents of Ferula communis L. subsp. communis growing spontaneously in Greece. Rec Nat Prod 7(1):54–58
Marongiu B, Piras A, Porcedda S (2005) Comparative analysis of the oil and supercritical CO2 extract of Ferula communis L. J Essent Oil Res 17:150–152
Massada Y (1976) Analysis of essential oils by gas chrotography and mass spectrometry. Hirokawa Publication, Tokyo, pp 43–285
Nagatsu A, Isaka K, Kojima K, Ondognii P, Zevgeegiin O, Gombosurengyin P, Davgiin K, Irfan B, Iquibal CM, Ggihara Y (2002) New sesquiterpenes from Ferula ferulaeoids (stard.) korovin.VI. Isolation and identification of three new dihyrofuro [2,3-b] chromones. Chem Pharm Bull 50(5):675–677
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Nicholas Au, Rettie AE (2008) Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metab Rev 40(2):355–375
Orhan I, Kartal M, Kan Y, Sener B (2008) Activity of essential oils and individual components against acetyl- and butyrylcholinesterase. Z Naturforsch C 63:547–553
Oyaizu M (1986) Studies on products of browning reaction: antioxidant activities of products of browning reaction prepared from glucosamine. Jpn J Nutr 44:307–315
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rubiolo P, Matteodo M, Riccio G, Ballero M, Christen P, Fleury-Souverain S, Veuthey JL, Bicchi C (2006) Analytical discrimination of poisonous and non poisonous chemotypes of giant fennel (Ferula communis L.) through their biologically active and volatile fractions. Agric Food Chem J 54:7556–7563
Rustaiyan A, Monfared A, Masoudi S (2001a) The essential oil of Ferula flabelliloba Rech. F. Essent Oil Res J 13:403–404
Rustaiyan A, Assadian F, Monfared A, Masoudi S, Yari M (2001b) Composition of the volatile oil of Ferula stenocarpa Boiss & Hausskn. Essent Oil Res J 13:181–182
Sayyah M, Kamalinejad M, Hidage RB, Rustaiyan A (2001) Antiepileptic potential and composition of the fruit essential oil of Ferula gummosa boiss. Iran Biomed J 5:69–72
Smail A, Lyoussi B, Miguel MG (2011) Antioxidant and anticholinesterase activities of some commercial essential oils and their major compounds. Molecules 16:7672–7690
Stenhagen E, Abrahamsson S, McLafferty FW (1974) Registry of mass spectral data. Wiley, New York
Stravroula M, Olga T, Artemios Y (2013) Volatile constituents of Ferula communis L. subsp. communis growing spontaneously. Rec Nat Prod J 7:54–58
Swigar AA, Silverstein RM (1981) Monoterpenes. Aldrich Chem Comp, Milwaukee
Tsai JY, Lee MJ, Dah-Tsyr Chang M, Huang H (2014) The effect of catalase on migration and invasion of lung cancer cells by regulating the activities of cathepsin S, L, and K. Exp Cell Res 323(1):28–40
Valle MG, Appendino G, Nano GM, Picci V (1987) Prenylated coumarins and sesquiterpenoids from F. communis. Phytochemistry 26:253–256
Yan R, Yang Y, Zeng Y (2009) Cytotoxicity and antibacterial activity of Lindera strychnifolia essential oils and extracts. Ethnopharmacol J 121:451–455
Yu F, Haradab H, Yamasakia K, Okamotoa S, Hirasec S, Tanakac Y, Misawab N, Utsumia R (2008) Isolation and functional characterization of a β-eudesmol synthase, a new sesquiterpene synthase from Zingiber zerumbet Smith. FEBS Lett 582:565–572
Znati M, Jabrane A, Hajlaoui H, Harzallah-Skhiri F, Bouajila J, Casanova J, Ben Jannet H (2012) Chemical composition and in vitro evaluation of antimicrobial and anti-acetylcholinesterase properties of the flower oil of Ferula lutea. Nat Prod Commun 7(7):947–950
Acknowledgments
Thanks are due to Dr. Fethia Harzallah SKHIRI (Higher Institute of Biotechnology of Monastir, Tunisia) for the botanical identification, and Dr. Ali OTHMANE (Biophysics Laboratory, Faculty of Medicine, Monastir), and Dr. Maha MASTOURI (Laboratory of Microbiologie, Fattouma BOURGUIBA Hospital, Monastir, Tunisia) for their help in performing the cytotoxic and antimicrobial tests, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Nguir, A., Mabrouk, H., Douki, W. et al. Chemical composition and bioactivities of the essential oil from different organs of Ferula communis L. growing in Tunisia. Med Chem Res 25, 515–525 (2016). https://doi.org/10.1007/s00044-016-1506-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-016-1506-1