Abstract
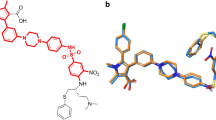
B-cell lymphoma extra large (Bcl-xL) is a member of the Bcl-2 family of proteins. This family has been implicated in the survival of cancer cells. Bcl-xL is known to be over-expressed in hematopoietic disorders and various types of cancers. The purpose of the present study is to develop an in silico model allowing for the identification of structural features influencing the inhibitory activity of Bcl-xL protein based on a diverse dataset of 104 compounds. Molecular docking studies are carried out to explore the binding of structurally diverse inhibitors to Bcl-xL by AutoDock and GOLD programs. The outcome of this investigation establishes the molecular basis for inhibition of Bcl-xL. Most of the compounds docked into the active site of Bcl-xL (PDB code 1R2D) with good docking scores and binding mode. The docking analysis of the highest active molecules—compound 50 (AutoDock binding affinity −10.8) and compound 100 (GoldScore 53.0)—from both the docking programs showed significant interaction with active site amino acid residues and H-bond interactions with the key amino acid residues. Current study identified a novel binding site of Bcl-xL protein. Forty compounds bound to this new binding site which is different from the formerly characterized hydrophobic pocket. The energy values of these potent compounds are also comparable with those binding to the previously reported binding site. This finding will help the researchers in the design of a better drug for the treatment of cancers.






Similar content being viewed by others
References
Becker OM, Dhanoa DS, Marantz Y, Chen D, Shacham S, Cheruku S, Heifetz A, Mohanty P, Fichman M, Sharadendu A, Nudelman R, Kauffman M, Noiman S (2006) An integrated in silico 3D model-driven discovery of a novel, potent, and selective amidosulfonamide 5-HT1A agonist (PRX-00023) for the treatment of anxiety and depression. J Med Chem 49:3116–3135
Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, Guo R, Li B, Zhu X, Huang Y, Long YQ, Roller PP, Yang D, Wang S (2001) Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem 44:4313–4324
Guillemin Y, Lopez J, Gimenez D, Fuertes G, Valero JG, Blum L, Gonzalo P, Salgado J, Girard-Egrot A, Aouacheria A (2010) Active fragments from pro- and antiapoptotic BCL-2 proteins have distinct membrane behavior reflecting their functional divergence. PLoS One 5:e9066
Hamsa TP, Kuttan G (2011) Harmine activates intrinsic and extrinsic pathways of apoptosis in B16F-10 melanoma. Chin Med 6:11
Hubbard RE, Haider MK (2010) Hydrogen bonds in proteins: role and strength. Wiley, Chichester
Jones G, Willett P, Glen RC (1995) Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J Mol Biol 245:43–53
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267:727–748
Kang MH, Reynolds CP (2009) Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res 15:1126–1132
Kutzki O, Park HS, Ernst JT, Orner BP, Yin H, Hamilton AD (2002) Development of a potent Bcl-x(L) antagonist based on alpha-helix mimicry. J Am Chem Soc 124:11838–11839
Kuwana T, Newmeyer DD (2003) Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol 15:691–699
Lavrado J, Moreira R, Paulo A (2010) Indoloquinolines as scaffolds for drug discovery. Curr Med Chem 17:2348–2370
Li Z, Wan H, Shi Y, Ouyang P (2004) Personal experience with four kinds of chemical structure drawing software: review on ChemDraw, ChemWindow, ISIS/Draw, and ChemSketch. J Chem Inf Comput Sci 44:1886–1890
Lu JJ, Bao JL, Chen XP, Huang M, Wang YT (2012) Alkaloids isolated from natural herbs as the anticancer agents. Evid Based Complement Alternat Med 2012:485042
Lugovskoy AA, Degterev AI, Fahmy AF, Zhou P, Gross JD, Yuan J, Wagner G (2002) A novel approach for characterizing protein ligand complexes: molecular basis for specificity of small-molecule Bcl-inhibitors. J Am Chem Soc 124:1234–1240
Manion MK, O’Neil JW, Giedt CD, Kim KM, Zhang KY, Hockenbery DM (2004) Bcl-XL mutations suppress cellular sensitivity to Antimycin A. J Biol Chem 279:2159–2165
Masood A, Azmi AS, Mohammed RM (2011) Small molecule inhibitors of Bcl-2 family proteins of pancreatic cancer therapy. Cancers (Basel) 3:1527–1549
Michels J, Kepp O, Senovilla L, Lissa D, Castedo M, Kroemer G, Galluzzi L (2013) Functions of BCL-XL at the interface between cell death and metabolism. Int J Cell Biol 2013:705294
Minn AJ, Rudin CM, Boise LH, Thompson CB (1995) Expression of Bcl-xL can confer a multidrug resistance phenotype. Blood 86:1903–1910
Moroy G, Martin E, Dejaegere A, Stote RH (2009) Molecular basis for Bcl-2 homology 3 domain recognition in the Bcl-2 protein family. J Biol Chem 284:17499–17511
Petros AM, Nettesheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, Fesik SW (2000) Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis and biophysical studies. Protein Sci 9:2528–2534
Petros AM, Dinges J, Augeri DJ, Baumeister SA, Betebenner DA, Bures MG, Elmore SW, Hajduk PJ, Joseph MK, Landis SK, Nettesheim DG, Rosenberg SH, Shen W, Thomas S, Wang X, Zanze I, Zhang H, Fesik SW (2006) Discovery of a potent inhibitor of the antiapoptotic protein Bcl-xL from NMR and parallel synthesis. J Med Chem 49:656–663
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612
Reddy KK, Singh SK, Tripathi SK, Selvaraj C (2013) Identification of potential HIV-1 integrase strand transfer inhibitors: in silico virtual screening and QM/MM docking studies. SAR QSAR Environ Res 24:581–595
Reed JC, Pellecchia M (2005) Apoptosis-based therapies for hematologic malignancies. Blood 106:408–418
Roberts NA, Martin JA, Kinchington D, Broadhurst AV, Craig JC, Duncan IB, Galpin SA, Handa BK, Kay J, Kröhn A (1990) Rational design of peptide-based HIV proteinase inhibitors. Science 248:358–361
Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW (1997) Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275:983–986
Shore GC, Viallet J (2005) Modulating the Bcl-2 family of apoptosis suppressors for potential therapeutic benefit in cancer. Hematol Am Soc Hematol Educ Program 2005:226–230
Singh J, Chuaqui CE, Boriack-Sjodin PA, Lee WC, Pontz T, Corbley MJ, Cheung HK, Arduini RM, Mead JN, Newman MN, Papadatos JL, Bowes S, Josiah S, Ling LE (2003) Successful shape-based virtual screening: the discovery of a potent inhibitor of the type I TGFbeta receptor kinase (TbetaRI). Bioorg Med Chem Lett 13:4355–4359
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461
Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW (2008) ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res 68:3421–3428
Verdonk ML, Berdini V, Hartshorn MJ, Mooij WT, Muray CW, Taylor RD, Watson P (2004) Virtual screening using protein-ligand docking: avoiding artificial enrichment. J Chem Inf Comput Sci 44:793–806
Yip KW, Reed JC (2008) Bcl-2 family proteins and cancer. Oncogene 27:6398–6406
Zhang YH, Bhunia A, Wan KF, Lee MC, Chan SL, Yu VC, Mok YK (2006) Chelerythrine and sanguinarine dock at distinct sites on BclXL that are not the classic BH3 binding cleft. J Mol Biol 364:536–549
Acknowledgments
Authors are highly grateful to Higher Education Commission Islamabad, Pakistan for funding this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azam, S.S., Abro, A., Tanvir, F. et al. Identification of unique binding site and molecular docking studies for structurally diverse Bcl-xL inhibitors. Med Chem Res 23, 3765–3783 (2014). https://doi.org/10.1007/s00044-014-0957-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-0957-5