Abstract
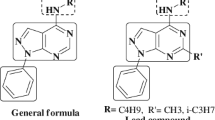
A series of 2-(4-cyano-3-trifluoromethylphenyl amino)-4-(4-quinazolinyloxy)-6-piperazinyl(piperidinyl)-s-triazines have been synthesized in this study by a simple and efficient synthetic protocol. The synthetic route to final piperazinyl s-triazines involved two nucleophilic substitution reactions of 4-amino-2-trifluoromethyl-benzonitrile and 4-hydroxyquinazoline with 2,4,6-trichloro-1,3,5-triazine resulting in 2,4-disubstituted-6-chloro-1,3,5-triazine derivative to introduce the piperazinyl or piperidinyl functionality. The structures of the compounds were elucidated with the aid of IR, 1H NMR, 13C NMR, 19F NMR spectroscopy, and elemental analysis. The antimicrobial activity of the compounds was tested against eight bacteria (Staphylococcus aureus MTCC 96, Bacillus cereus MTCC 619, Escherichia coli MTCC 739, Pseudomonas aeruginosa MTCC 741, Klebsiella pneumoniae MTCC 109, Salmonella typhi MTCC 733, Proteus vulgaris MTCC 1771, Shigella Flexneria MTCC 1457) and four fungi (Aspergillus niger MTCC 282, Aspergillus fumigatus MTCC 343, Aspergillus clavatus MTCC 1323, and Candida albicans MTCC 183). The title compounds were also investigated for their antituberculosis activity against MTB H37 RV strain using BACTEC MGIT and L. J. agar dilution method. The bioassay results showed that compounds 5d, 5n, 5p, 5s, and 5t demonstrated 99% inhibition at the MIC of 6.25 μg/ml, equivalent to standard drug pyrazinamide.
Similar content being viewed by others
Introduction
A higher incidence of opportunistic microbial infections caused by various bacteria and fungi due to the evolution and spread of multidrug-resistant microorganisms has become a widespread medical problem. Such infections most commonly cause severe morbidity and mortality in debilitated and immunocompromised patients (Nathan, 2004). Such infections most commonly affect immunocompromised individuals, patients with malignancies, and transplant recipients. Moreover, with an indication of around two million deaths each year, TB remains a serious epidemiological problem, establishes mainly in the pulmonary system, and is caused by some mycobacteria of the Mycobacterium tuberculosis complex, predominantly Mycobacterium tuberculosis (Raviglione, 2003). According to the World Health Organization (WHO), in 2010, there were 8.8 million (range, 8.5–9.2 million) incident cases of TB, 1.1 million (range, 0.9–1.2 million) deaths from TB among HIV-negative people, and an additional 0.35 million (range, 0.32–0.39 million) deaths from HIV-associated TB (WHO, 2011). These facts further underscore the urgent necessity to find new efficacious and safe compounds to maintain and improve the management and prevention of opportunistic microbial and tubercular infections in a new era of intensive infectious disease control, elimination, and eradication.
1,3,5-Triazine derivatives are implicated in a variety of biological applications, such as antimicrobial (Chikhalia and Patel, 2009; Patel et al., 2010a, b; Srinivas et al., 2006; Zhou et al., 2008), antiprotozoal (Baliani et al., 2005), anticancer (Menicagli et al., 2004), antimalarial (Sergio et al., 2008), and antiviral (Mahajan et al., 2009; Xiong et al., 2008) activities. In addition, piperazines and substituted piperazines are a well-established class of heterocyclic compounds attracting significant interest in medicinal chemistry (Kerns et al., 2003; Kumar et al., 2008; Upadhayaya et al., 2004). Recently several s-triazine derivatives bearing morpholine, piperidine, and some piperazine moieties are characterized by an enhanced antibacterial profile and improved pharmacokinetic properties as antitubercular activity (Sunduru et al., 2010). The hydroxyl-substituted quinazoline derivatives exhibit a wide variety of biological activities (Nosova et al., 2009; Karminski et al., 1983; Tran et al., 2004; Tsizin et al., 1973), and as a consequence of large therapeutic potential of such biolabile analogues, it was rationalized to synthesize newer structural elements potentially endowed with antibacterial and antifungal activities.
We have recently described the synthesis and the biological activity of some s-triazine analogues incorporating 4-amino-benzonitrile, 4-amino-2-trifluoromethyl-benzonitrile, and various substituted hydroxyl quinoline moieties (Patel et al., 2010a, b; Patel et al., 2011a, b, c), and the compounds have demonstrated promising antimicrobial activity, representing a promising lead for further optimization. In order to extend their structure–activity relationships (SARs), we have designed and synthesized a novel series based 4-amino-2-trifluoromethyl-benzonitrile and 4-hydroxyquinazole incorporated s-triazines motivated by the aforementioned findings. We have introduced the similar piperazine bases to both of the systems in an order, to identify the difference between the biological profiles of the resultant series, in which activity was found to be increased significantly against most of studied strains of bacteria and fungi in terms of MIC as well as zone of inhibition. In this article, we report the further evolution of that previous exploration aimed at identifying additional clinical candidates that exhibit appropriate drug-like antimicrobial properties.
Results and discussion
Chemistry
Compounds 5a–5u were synthesized according to Fig. 1. The disubstituted s-triazine intermediate 3 was obtained in very good yield by the reaction between 4-(4,6-dichloro-1,3,5-triazin-2-ylamino)-2-trifluoromethyl-benzonitrile (1) and 4-hydroxyquinazoline in the presence of 60% NaH at 45–50°C. Nucleophilic substitution of one chlorine atom of s-triazine ring produced 1 in good yield from 2,4,6-trichloro-1,3,5-triazine and 4-amino-2-trifluoromethyl-benzonitrile. Condensation of 3 with appropriate piperazine and piperidine substituents in 1,4-dioxane at 70–80°C provided the target compounds 5a–5u. A C3N3 stretching in the s-triazine ring was observed at 810–820 cm−1. Compound 1 displayed an absorption band at 2,218–2,225 cm−1, confirming the presence of a –C≡N group, and a strong band near 3,250–3,295 cm−1 due to the presence of an –NH group. Moreover, a characteristic band appeared at 1,248–1,260 cm−1 corresponding to the C–O–C linkage, while disappearance of the –OH peak at 3,606–3,632 cm−1, belonging to the 4-hydroxyquinazoline, gave correction to the formation of intermediate 3. The absence of a C–Cl stretching band at 700–760 cm−1 confirmed the formation of the final products by the condensation of piperazines to s-triazine ring as all the chlorine atoms of s-triazine ring were substituted by 4-aminol-benzonitrile, 4-hydroxyquinazoline, and piperazines or piperidines. The synthesis of 5a–5u was confirmed on the basis of NMR spectra. The piperazine proton assigned a signal at 3.39–3.52 ppm integrating four proton atoms, as well as at 3.79–3.89 ppm integrating remaining four proton atoms, some of the proton atoms corresponding to the quinazoline nucleus moiety resonated at 7.70–8.60 ppm region, the –NH group at 9.70–9.95 ppm. 13C NMR spectral-assigned signals in the range 171–173, 168–170, and 165–167 ppm attributed to the carbon atoms of s-triazine ring from which the chlorine atoms were replaced by 4-amino-2-trifluoromethyl-benzonitrile, 4-hydroxyquinazoline and piperazines, or piperidines. The carbon atoms nearer to the nitrogen heteroatom in the quinazoline ring integrated at 155–160 ppm. In addition, all the final analogues with single fluorine-substituted piperazine coupling bases gave a peak at around 150–152 ppm corresponding to the carbon–fluorine-bounded carbon atom of the phenyl ring of piperazine base. Furthermore, two frequencies observed at around 130 and 125 ppm interpreted as attributable to the trifluoromethyl functional group and the carbon atom to which the trifluoromethyl group is attached, respectively. The carbon atom corresponding to the cyano functional group (C≡N) and the carbon atom of the amino benzonitrile ring to which the cyano group is attached were found to reveal the peaks at around 105 and 96–98 ppm, respectively. The carbon atoms of the piperazine ring gave signals in the range of 46–50 ppm. 19F NMR spectra for the analogues 5q and 5r confirmed the presence of fluorine atoms in the ortho- and para-positions of the phenyl ring, respectively, by forming the corresponding peaks at around −124 and −119 ppm, whereas, the same compounds revealed another singlet peak in their 19F NMR spectra at −67 and −65 ppm corresponding to the presence of three fluorine atoms in trifluoromethyl functionalized amino-benzonitrile moiety. 19F NMR spectra obtained for the compound 5s gave two singlets at −66.9 and −66.1 ppm corresponding to the presence of two trifluoromethyl functional groups in the meta-position of the phenyl ring of piperazine base condensed to s-triazine nucleus as well as at amino-benzonitrile moiety. All of the novel compounds gave C, H, and N analyses with their values being within 0.4 percent points from the theoretical values, i.e., within the acceptable range (Figs. 1, 2).
Antimicrobial activity
The antimicrobial bioassay results presented in Table 1 revealed that, generally, all the tested compounds tended to be more active against gram-positive bacteria than against gram-negative bacteria. Final s-triazinyl compounds 5d, 5s. and 5t showed potent activity (MIC, 3.12 μg/ml) against gram-positive strain S. aureus. Compounds 5n, 5p, 5q, 5r. and 5u were found to possess half-fold activity (MIC, 6.25 μg/ml) against S. aureus as compared to the most active analogues tested against the same strain, in which compound 5p exhibited similar zone of inhibition (29 mm) as the most active derivatives. Final s-triazinyl analogues 5s (28 mm of zone of inhibition) and 5t (27 mm of zone of inhibition) displayed excellent inhibitory profile at 3.12 μg/ml against gram-positive B. cereus along with half-fold comparative activity of compounds 5h, 5p, 5q. and 5u (MIC, 6.25 μg/ml) against the same bacterial strain. Compounds 5h, 5t. and 5u (28 mm of zone of inhibition) were found to contribute promising activity (MIC, 6.25 μg/ml) along with similar inhibitory concentration level of compound 5n (27 mm of zone of inhibition) toward gram-negative strain E. coli, while compound 5i demonstrated 12.5 μg/ml of inhibitory concentration level against E. coli. Compounds 5p, 5r. and 5s appeared with diminished activity against gram-negative P. aeruginosa at 6.25 μg/ml of MIC, where the half-fold activity was observed (MIC, 12.5 μg/ml) for compounds 5h, 5n. and 5u against the same bacteria. Inhibition of gram-negative bacteria K. pneumoniae was also noted for s-triazine derivatives 5n and 5t at 6.25 μg/ml as well as 5s with quite reduced zone diameter (25 mm). Another two derivatives denoted by 5h and 5u were reported to exhibit half-fold growth inhibition of the same bacteria (MIC, 12.5 μg/ml). Compound 5p and 5r (27 mm of zone of inhibition), and compound 5c (26 mm of zone of inhibition) possessed the highest activity (MIC, 6.25 μg/ml) against gram-negative S. typhi along with half-fold activity profile of compounds 5d and 5q (MIC, 12.5 μg/ml). Final s-triazine derivatives 5d, 5n, 5s, and 5u (26 mm of zone of inhibition) and 5t (25 mm of zone of inhibition) were superior in inhibiting the growth of gram-negative P. vulgaris (MIC, 6.25 μg/ml), whereas half-fold activity was observed in case of compounds 5h and 5r at 12.5 μg/ml. Compounds 5c and 5s were found to be effective (27 mm of zone of inhibition) in controlling the growth of gram-negative bacteria S. flexneria (MIC, 12.5 μg/ml) along with similar efficacy of compound 5d (MIC, 12.5 μg/ml) against the same bacteria with quite reduced diameter of zone of inhibition (26 mm). Half-fold anti S. flexneria activity (MIC, 25 12.5 μg/ml) was noted for the compound 5q. All the remaining final s-triazine derivatives were found to demonstrate good-to-poor activity profiles at minimum inhibitory concentration levels ranging from 25 to 100 μg/ml, whereas some final derivatives were found to be inactive even at a higher concentration of 100 μg/ml.

The antifungal bioassay results summarized in Table 2 revealed that final s-triazine derivatives 5q and 5s displayed excellent antigrowth activity (MIC, 3.12 μg/ml) against A. niger, which was found equivalent to the standard drug tested. Compounds, 5d, 5t, and 5u, appeared with half-fold inhibitory action against the same fungi at MIC, 6.25 μg/ml. Antifungal screening was carried out on A. fumigates among the tested compounds which showed that compounds 5t and 5u contributed the highest inhibition at 6.25 μg/ml of MIC, whereas 5c, 5p, 5r, and 5s demonstrated half-fold activity (12.5 μg/ml). The growth of A. clavatus fungi was inhibited strongly by compounds 5n, 5s, 5t, and 5u (MIC, 6.25 μg/ml), while compounds 5h, 5p, and 5r were found to display half-fold MIC of 12.5 μg/ml. Compounds 5d, 5r, and 5s appeared with strong inhibition of C. albicans at 6.25 μg/ml. Compounds 5c, 5t, 5u, and 5q indicated half-fold activity (12.5 μg/ml) compared to the most active analogues toward C. albicans. Remaining final compounds were found to contribute good-to-poor activities against all the mentioned fungal strains at the concentration levels ranging from 25 to 100 μg/ml, whereas some final derivatives were found to be inactive even at a higher concentration of 100 μg/ml.

Antituberculosis activity
In vitro antituberculosis results observed for final analogues (5a–5u) from BACTEC MGIT method (Table 3) indicated that final s-triazines, 5d bearing two electron-withdrawing chlorine groups incorporated into the 2nd and 3rd positions of the phenyl ring of piperazine base; 5n with two electron-releasing methyl groups to the 3rd and 5th positions of the piperidines moiety; 5p bearing mono-halo-substituted N-benzhydryl piperazine base; 5s bearing trifluoromethyl group attached to the meta-position of phenyl ring of piperazine base; and 5t with three methoxy groups introduced into the ortho-, meta-, and para-positions of the phenyl ring of piperazine base, condensed to s-triazine nucleus exhibited excellent inhibition (99%) at 6.25 μg/ml against mycobacterial strain. These compounds were considered to be the most potent analogues among all the final compounds studied. The primary BACTEC MGIT bioassay results obtained have driven us to examine the potency (MIC) of the remaining compounds against M. tuberculosis H37Rv. In the secondary biological screening (L. J agar dilution method), it was observed that final s-triazinyl compound 5h, involving insertion of piperazine moiety containing acetyl linkage, 5q and 5r, incorporating mono-halo (fluoro)-substituted phenyl ring of piperazine entity bridged to s-triazine core, displayed good inhibition effect with the MIC of 12.5 μg/ml, i.e., half-fold activity than the most active analogues tested. Compounds 5c and 5u, incorporating single chlorine and methoxy group to the phenyl ring of piperazine base condensed to nucleus, demonstrated 25 μg/ml of MIC against M. tuberculosis H37Rv. Final s-triazine derivatives with N-isopropyl piperazine constituent, 5i, as well as N-benzhydryl piperazine constituent, 5o, indicated lower inhibitory action at the MIC, 50, and 62.5 μg/ml, respectively. Final morpholine-bearing compound 5e displayed moderate inhibition of M. tuberculosis H37RV at the MIC level of 100 μg/ml, whereas some derivatives were found to be inactive even at a higher concentration of 100 μg/ml.

Experimental
Materials
Melting points were determined in open capillaries on a Veego electronic apparatus VMP-D and are uncorrected. IR spectra (4,000–400 cm−1) of the synthesized compounds were recorded on a Shimadzu 8400-S FT-IR spectrophotometer. Thin layer chromatography was performed on object glass slides (2 × 7.5 cm) coated with silica gel-G, and spots were visualized under UV irradiation. NMR spectra were recorded on a Varian 400-MHz spectrometer using DMSO (dimethyl sulfoxide) as solvent and TMS (tetramethylsilane) as an internal standard. Elemental analyses (C, H, and N) were performed using a Heraeus Carlo Erba 1180 CHN analyzer.
Synthesis part
4-[4,6-Dichloro-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (1) was synthesized according to the previously reported literature (Patel et al., 2011a, b, c).
4-[4-Chloro-6-(quinazoline-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethy benzonitrile (3). To a stirred solution of 8 g, 0.055 mol of 4-hydroxyquinazoline in anhydrous THF, 1.32 g, 0.055 mol of 60% NaH was added at room temperature during 1 h, and equivalent quantity of 1 (14.66 g) was then added to the mixture, and stirred for another 20 h at 45°C. After the completion of the reaction, monitored by TLC using toluene:acetone (95:5 v/v) as eluent, the mixture was treated with crushed ice, filtered, and dried using vacuum pump to obtain 3 [Xiong et al., 2009]. Yield: 84%, m.p. 257°C (dec.). IR (KBr) cm−1: 2,223 (C≡N), 1,255–1,257 (C–O–C).
General procedure for the synthesis of compounds (5a–5u)
To a stirred solution of 0.01 mol of intermediate 3 in 30 ml of 1,4-dioxane, the equivalent quantity of each piperazine or piperidine derivative was added, and the mixture was refluxed for 12–16 h using K2CO3 for neutralization. Thin Layer Chromatography was used for monitoring the progress of reaction using toluene:acetone (95:5 v/v) as eluent. After the completition of the reaction, the mixture was poured into crushed ice and neutralized by diluted HCl. The precipitate thus obtained was filtered off, dried, and recrystallized from THF to give the final compounds, 5a–5u (Patel et al., 2010a, b).
4-[4-(4-Methyl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5a). Yield: 78%. m.p. 273–275°C (THF). IR (KBr) cm−1: 3,250 (–NH), 2,221 (C≡N), 1,255 (C–O–C), 814 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.57 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.55 (s, 1H, N–CH–N of quinazoline ring), 8.11 (dd, J = 10.4, 7.9 Hz, 2H, quinazoline ring), 7.76 (t, J = 7.8 Hz, 1H, quinazoline ring), 7.46–7.37 (m, 6H, Ar–H), 3.89 (br s, 4H, piperazine ring), 3.40 (br s, 4H, piperazine ring), 1.97 (s, 1H, N–CH 3). 13C NMR (100 MHz, DMSO-d6): δ = 173.12 (C-6, s-triazine, C–N at piperazine linkage), 169.21 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.40 (C-4, s-triazine, C–O–C at quinazoline linkage), 159.11, 158.88, 156.19 (3C, Quinazoline heteroatom ring carbon atoms), 149.80–121.35 (11C, Ar–C), 105.11 (1C, C≡N), 98.90 (1C, –C–C≡N), 50.37, 47.04 (4C of piperazine ring carbon atoms), 21.59 (1C, –CH3). Anal. Calcd. for C24H20F3N9O: C, 56.80; H, 3.97; N, 24.84. Found: C, 56.48; H, 3.73; N, 25.08.
4-[4-(4-Ethyl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5b). Yield: 83%. m.p. 247–248°C (THF). IR (KBr) cm−1: 3,276 (–NH), 2,223 (C≡N), 1,255 (C–O–C), 806 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): 9.70 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.51 (s, 1H, N–CH–N of quinazoline ring), 8.20 (dd, J = 10.8, 7.2 Hz, 2H, quinazoline ring), 7.71 (t, J = 7.0 Hz, 1H, quinazoline ring), 7.53–7.39 (m, 4H, Ar–H), 3.89 (br s, 4H, piperazine ring), 3.39 (br s, 4H, piperazine ring), 2.48 (q, J = 6.7 Hz, 2H, N–CH 2–CH3), 1.90 (t, J = 6.1 Hz, 3H, CH2–CH 3). 13C NMR (100 MHz, DMSO-d6): δ = 172.7 (C-6, s-triazine, C–N at piperazine linkage), 168.4 (C-2, s-triazine, C–NH at benzonitrile moiety), 165.3 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.7, 157.8, 156.9 (3C, Quinazoline heteroatom ring carbon atoms), 148.4–121.6 (9C, Ar. C), 105.1 (1C, C≡N), 96.8 (1C, –C–C≡N), 47.97, 46.7, 43.6 (5C, 4C of piperazine ring carbon atoms and 1C of N–CH2), 14.7 (1C, CH2–CH3). Anal. Calcd. for C25H22F3N9O: C, 57.58; H, 4.25; N, 24.17. Found: C, 57.26; H, 4.54; N, 24.31.
4-[4-[4-(3-Chloro-phenyl)-piperazin-1-yl]-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5c). Yield: 79%. m.p. 262–264°C (THF). IR (KBr) cm−1: 3,270 (–NH), 2,223 (C≡N), 1,257 (C–O–C), 813 (s-triazine C–N str.), 754 (C–Cl). 1H NMR (400 MHz, DMSO-d6): δ = 9.46 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.43 (s, 1H, N–CH–N of quinazoline ring), 8.07 (dd, J = 11.2, 7.5 Hz, 2H, quinazoline ring), 7.79 (t, J = 7.6 Hz, 1H, quinazoline ring), 7.41–7.25 (m, 5H, Ar–H), 7.04 (dd, J = 7.8, 6.7 Hz, 1H), 6.73 (dd, J = 11.1, 4.4 Hz, 1H), 6.60 (d, J = 7.3 Hz, 1H), 3.82 (br s, 4H, piperazine ring), 3.49 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 173.1 (C-6, s-triazine, C–N at piperazine linkage), 169.6 (C-2, s-triazine, C–NH at benzonitrile moiety), 167.7 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.8, 158.3, 157.10 (3C, Quinazoline heteroatom ring carbon atoms), 151.0–124.7 (17C, Ar. C), 104.9 (1C, C≡N), 99.0 (1C, –C–C≡N), 49.2, 47.2 (4C, piperazine ring carbon atoms). Anal. Calcd. for C29H21ClF3N9O: C, 57.67; H, 3.50; N, 20.87. Found: C, 57.42; H, 3.78; N, 21.13.
4-[4-[4-(2,3-Dichloro-phenyl)-piperazin-1-yl]-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5d). Yield: 71%. m.p. >300°C (THF). IR (KBr) cm−1: 3,263 (–NH), 2,221 (C≡N), 1,255 (C–O–C), 806 (s-triazine C–N str.), 754 (C–Cl). 1H NMR (400 MHz, DMSO-d6): δ = 9.64 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.56 (s, 1H, N–CH–N of quinazoline ring), 8.21 (dd, J = 10.9, 7.8 Hz, 2H, quinazoline ring), 7.69 (t, J = 7.0 Hz, 1H, quinazoline ring), 7.32–7.27 (m, 2H, Ar–H), 7.16 (d, J = 7.4 Hz, 2H), 7.04–6.97 (m, 1H), 6.83 (t, J = 7.3 Hz, 1H), 6.54 (dd, J = 7.0, 0.8 Hz, 1H), 3.85 (br s, 4H, piperazine ring), 3.38 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 173.5 (C-6, s-triazine, C–N at piperazine linkage), 169.6 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.5 (C-4, s-triazine, C–O–C at quinazoline linkage), 159.0, 158.8, 155.4 (3C, Quinazoline heteroatom ring carbon atoms), 150.7–120.2 (17C, Ar. C), 104.9 (1C, C≡N), 97.1 (1C, –C–C≡N), 49.6, 45.2 (4C, piperazine ring carbon atoms). Anal. Calcd. for C29H20Cl2F3N9O: C, 54.56; H, 3.16; N, 19.75. Found: C, 54.24; H, 3.43; N, 19.61.
4-[4-Piperidin-1-yl-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5e). Yield: 80%. m.p. 288–291°C (Acetone). IR (KBr) cm−1: 3,287 (–NH), 2,223 (C≡N), 1,256 (C–O–C), 817 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.69 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.46 (s, 1H, N–CH–N of quinazoline ring), 8.02 (dd, J = 10.1, 7.8 Hz, 2H, quinazoline ring), 7.79 (t, J = 7.1 Hz, 1H, quinazoline ring), 7.60–7.41 (m, 6H, Ar–H), 3.82 (t, J = 4.9 Hz, 4H, piperidine), 3.71 (t, J = 4.6 Hz, 4H, piperidine). 13C NMR (100 MHz, DMSO-d6): δ = 171.9 (C-6, s-triazine, C–N at piperazine linkage), 169.7 (C-2, s-triazine, C–NH at benzonitrile moiety), 168.6 (C-4, s-triazine, C–O–C at quinazoline linkage), 156.2, 155.4, 155.0 (3C, Quinazoline heteroatom ring carbon atoms), 148.2–118.0 (12C, Ar. C), 105.0 (1C, C≡N), 97.9 (1C, –C–C≡N), 56.7, 46.4 (4C, morpholine ring carbon atoms). Anal. Calcd. for C24H19F3N8O: C, 58.53; H, 3.89; N, 22.75. Found: C, 58.41; H, 4.04; N, 22.48.
4-[4-Morpholin-4-yl-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5f). Yield: 90%. m.p. 275–276°C (THF). IR (KBr) cm−1: 3,259 (–NH), 2,221 (C≡N), 1,375 (Morpholine C–O–C str.), 1,258 (C–O–C), 806 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): 9.56 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), δ 8.53 (s, 1H, N–CH–N of quinazoline ring), 8.16 (dd, J = 9.9, 8.5 Hz, 2H, quinazoline ring), 7.90 (t, J = 6.5 Hz, 1H, quinazoline ring), 7.46 (d, J = 7.1 Hz, 2H), 2.49–2.46 (m, 4H, CH2 piperidinyl), 1.46–1.41 (m, 4H, CH2 piperidinyl), 1.33–1.29 (m, 2H, CH2 piperidinyl). 13C NMR (100 MHz, DMSO-d6): δ = 172.7 (C-6, s-triazine, C–N at piperazine linkage), 169.6 (C-2, s-triazine, C–NH at benzonitrile moiety), 165.7 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.3, 157.8, 156.2 (3C, Quinazoline heteroatom ring carbon atoms), 147.4–119.7 (10C, Ar. C), 105.2 (1C, C≡N), 97.1 (1C, –C–C≡N), 40.6, 30.8, 27.7 (5C, C-26 to C-30, piperidine ring carbon atoms). Anal. Calcd. for C23H17F3N8O2: C, 55.87; H, 3.47; N, 22.66. Found: C, 56.09; H, 3.67; N, 22.37.
4-[4-(4-Phenyl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5g). Yield: 81%. m.p. 256–258°C (DMF). IR (KBr) cm−1: 3,277 (–NH), 2,223 (C≡N), 1,256 (C–O–C), 815 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.54 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.47 (s, 1H, N–CH–N of quinazoline ring), 8.14–8.09 (m, 2H, quinazoline ring), 7.74 (t, J = 6.9 Hz, 1H, quinazoline ring), 7.57–7.22 (m, 9H, Ar–H), 3.80 (br s, 4H, piperazine ring), 3.39 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 172.6 (C-6, s-triazine, C–N at piperazine linkage), 168.8 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.9 (C-4, s-triazine, C–O–C at quinazoline linkage), 159.8, 156.9, 155.10 (3C, Quinazoline heteroatom ring carbon atoms), 148.1–124.0 (17C, Ar. C), 104.95 (1C, C≡N), 97.8 (1C, –C–C≡N), 49.0, 47.6 (4C, piperazine ring carbon atoms). Anal. Calcd. for C29H22F3N9O: C, 61.16; H, 3.89; N, 22.13. Found: C, 61.39; H, 3.71; N, 22.53.
4-[4-(4-Acetyl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5h). Yield: 84%. m.p. 238–240°C (THF). IR (KBr) cm−1: 3,282 (–NH), 2,218 (C≡N), 1,700 (–C=O), 1,475 (–CH3), 1,255 (C–O–C), 819 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.51 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.29 (s, 1H, N–CH–N of quinazoline ring), 8.19 (dd, J = 10.7, 7.7 Hz, 2H, quinazoline ring), 7.83 (t, J = 7.1 Hz, 1H), 7.37–7.28 (m, 4H, Ar–H), 3.83 (br s, 4H, piperazine ring), 3.44 (br s, 4H, piperazine ring), 2.23 (s, 3H,COCH 3). 13C NMR (100 MHz, DMSO-d6): δ = 171.9 (C-6, s-triazine, C–N at piperazine linkage), 169.0, 168.2 (2C, 1C at C-2 of s-triazinyl C-NH at benzonitrile moiety and 1C at N–COCH3), 167.6 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.7, 158.3, 156.5 (3C, Quinazoline heteroatom ring carbon atoms), 147.5–120.8 (11C, Ar. C), 105.4 (1C, C≡N), 98.9 (1C, –C–C≡N), 50.1, 48.9 (4C, piperazine ring carbon atoms), 22.3 (COCH3). Anal. Calcd. for C25H20F3N9O2: C, 56.07; H, 3.76; N, 23.54. Found: C, 56.34; H, 3.51; N, 23.29.
4-[4-(4-Isopropyl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]]-2-trifluoromethyl-benzonitrile (5i). Yield: 67%. m.p. 287–288°C (THF). IR (KBr) cm−1: 3,251 (–NH), 2,224 (C≡N), 1,380 (isopropyl), 1,257 (C–O–C), 819 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.62 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.50 (s, 1H, N–CH–N of quinazoline ring), 8.21 (dd, J = 9.6, 8.4 Hz, 2H, quinazoline ring), 7.89 (t, J = 7.4 Hz, 1H, quinazoline ring), 7.60–7.35 (m, 10H, Ar–H), 3.83 (br s, 4H, piperazine ring), 3.42 (br s, 4H, piperazine ring), 2.73–2.68 (m, 1H, N–CH), 1.71 (d, J = 6.5 Hz, 6H, –2CH 3). 13C NMR (100 MHz, DMSO-d6): δ = 173.0 (C-6, s-triazine, C–N at piperazine linkage), 169.3 (C-2, s-triazine, C–NH at benzonitrile moiety), 167.7 (C-4, s-triazine, C–O–C at quinazoline linkage), 157.5, 156.8, 155.9 (3C, Quinazoline heteroatom ring carbon atoms), 149.5–116.4 (11C, Ar. C), 105.2 (1C, C≡N), 96.1 (1C, –C–C≡N), 51.1, 48.8, 43.2 (5C, 4C of piperazine ring carbon atoms and 1C of N–CH), 21.7 (2C, 2CH 3). Anal. Calcd. for C26H24F3N9O: C, 58.31; H, 4.52; N, 23.54. Found: C, 57.98; H, 4.67; N, 23.75.
4-[4-(4-Pyridin-2-yl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5j). Yield: 80%. m.p. 291–293°C (THF). IR (KBr) cm−1: 3,292 (–NH), 2,221 (C≡N), 1,256 (C–O–C), 806 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.49 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.44 (s, 1H, N–CH–N of quinazoline ring), 8.20–8.14 (m, 2H), 8.04 (d, J = 7.3 Hz, 1H, N–CH, pyridyl), 7.79 (t, J = 7.5 Hz, 1H, quinazoline ring), 7.47–7.32 (m, 6H, Ar–H), 6.70 (t, J = 7.5 Hz, 1H, pyrimidyl), 3.41 (br s, 4H, piperazine ring), 3.87 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 173.9 (C-6, s-triazine, C–N at piperazine linkage), 169.3 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.6 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.1, 157.9, 157.4, 154.5 (4C, Quinazoline and pyridyl heteroatom ring carbon atoms), 149.5–119.3 (14C, Ar. C), 104.9 (1C, C≡N), 97.0 (1C, –C–C≡N), 49.2, 45.0 (4C, piperazine ring carbon atoms). Anal. Calcd. for C28H21F3N10O: C, 58.95; H, 3.71; N, 24.55. Found: C, 58.71; H, 3.90; N, 24.23.
4-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazin-2-ylamino]-2-trifluoromethyl-benzonitrile (5k). Yield: 85%. m.p. 283–284°C (THF). IR (KBr) cm−1: 3,286 (–NH), 2,220 (C≡N), 1,255 (C–O–C), 806 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.55 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.39 (d, J = 8.3 Hz, 1H, N–CH–N of quinazoline ring), 8.37 (d, J = 7.9 Hz, 2H, pyrimidyl ring proton atoms), 8.10 (dd, J = 11.4, 8.3 Hz, 2H, quinazoline ring), 7.70 (t, J = 6.8 Hz, 1H, quinazoline ring), 7.55–7.37 (m, 5H, Ar–H), 3.80 (br s, 4H, piperazine ring), 3.39 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 172.8 (C-6, s-triazine, C–N at piperazine linkage), 168.4 (C-2, s-triazine, C–NH at benzonitrile moiety), 165.6 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.3, 157.2, 156.32, 155.8, 154.0 (6C, Quinazoline and pyrimidyl heteroatom ring carbon atoms), 148.3–120.7 (12C, Ar. C), 104.7 (1C, C≡N), 99.1 (1C, –C–C≡N), 48.5, 47.6 (4C, piperazine ring carbon atoms). Anal. Calcd. for C27H20F3N11O: C, 56.74; H, 3.53; N, 26.96. Found: C, 56.95; H, 3.32; N, 26.63.
4-[4-(4-Benzyl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazine-2-ylamino]-2-trifluoromethyl-benzonitrile (5l). Yield: 69%. m.p. 268–269°C (THF). IR (KBr) cm−1: 3,255 (–NH), 2,221 (C≡N), 1,255 (C–O–C), 806 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.68 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.40 (s, 2H, N–CH–N of quinazoline ring), 8.08 (dd, J = 12.7, 4.6 Hz, 2H, quinazoline ring), 7.76 (t, J = 6.9 Hz, 2H, quinazoline ring), 7.50–7.19 (m, 7H, Ar–H), 3.88 (br s, 4H, piperazine ring), 3.45 (br s, 4H, piperazine ring), 2.93 (s, 2H, N–CH 2). 13C NMR (100 MHz, DMSO-d6): δ = 172.7 (C-6, s-triazine, C–N at piperazine linkage), 170.5 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.4 (C-4, s-triazine, C–O–C at quinazoline linkage), 157.7, 157.0, 155.9 (3C, Quinazoline heteroatom ring carbon atoms), 151.0–118.7 (17C, Ar. C), 105.6 (1C, C≡N), 96.9 (1C, –C–C≡N), 69.7 (1C, N–CH2), 50.6, 47.0 (4C, piperazine ring carbon atoms). Anal. Calcd. for C30H24F3N9O: C, 61.74; H, 4.15; N, 21.60. Found: C, 61.99; H, 3.82; N, 21.47.
4-[4-(4-Benzyl-piperidin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazine-2-ylamino]-2-trifluoromethyl-benzonitrile (5m). Yield: 72%. m.p. 287–289°C (THF). IR (KBr) cm−1: 3,260 (–NH), 2,223 (C≡N), 1,257 (C–O–C), 816 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.71 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.56 (s, 1H, N–CH–N of quinazoline ring), 8.20 (dd, J = 11.7, 5.7 Hz, 2H, quinazoline ring), 7.77 (t, J = 7.5 Hz, 1H, quinazoline ring), 7.61–7.32 (m, 9H, Ar–H), 3.76 (4H, t, J = 7.0 Hz, Piperidine), 3.61 (4H, t, J = 8.8 Hz, Piperidine), 2.44 (2H, s, –CH2), 1.73 (1H, t, J = 6.9 Hz, –CH, piperidine). 13C NMR (100 MHz, DMSO-d6): δ = 172.6 (C-6, s-triazine, C–N at piperazine linkage), 169.5 (C-2, s-triazine, C–NH at benzonitrile moiety), 167.3 (C-4, s-triazine, C–O–C at quinazoline linkage), 157.9, 156.4, 155.0 (3C, Quinazoline heteroatom ring carbon atoms), 150.0–124.3 (17C, Ar. C), 105.1 (1C, C≡N), 97.9 (1C, –C–C≡N), 41.5, 37.1, 35.9, 28.0 (6C, piperidine ring carbon atoms and –CH2). Anal. Calcd. for C31H25F3N8O: C, 63.91; H, 4.33; N, 19.23. Found: C, 63.70; H, 4.01; N, 19.50.
4-[4-(3,5-Dimethyl-piperidin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazine-2-ylamino]-2-trifluoromethyl-benzonitrile (5n). Yield: 81%. m.p. 289–290°C (DMF). IR (KBr) cm−1: 3,280 (–NH), 2,220 (C≡N), 1,257 (C–O–C), 808 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.54 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.32 (s, 1H, N–CH–N of quinazoline ring), 8.12 (dd, J = 11.9, 5.4 Hz, 2H, quinazoline ring), 7.85 (t, J = 6.5 Hz, 1H, quinazoline ring), 7.59–7.40 (m, 5H, Ar–H), 3.64 (dd, J = 12.7, 7.4 Hz, 2H, piperidine), 3.13 (dd, J = 12.1, 7.3 Hz, 2H, piperidine), 1.80–1.77 (m, 3H, piperidine), 1.41 (d, J = 6.7 Hz, 6H, 2CH 3). 13C NMR (100 MHz, DMSO-d6): δ = 173.0 (C-6, s-triazine, C–N at piperazine linkage), 168.6 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.2 (C-4, s-triazine, C–O–C at quinazoline linkage), 159.0, 157.8, 156.68 (3C, Quinazoline heteroatom ring carbon atoms), 149.7–120.3 (11C, Ar. C), 104.93 (1C, C≡N), 96.9 (1C, –C–C≡N), 52.0, 44.5, 32.3 (5C, piperidine), 22.8 (2C, 2CH3). Anal. Calcd. for C26H23F3N8O: C, 59.99; H, 4.45; N, 21.53. Found: C, 60.16; H, 4.20; N, 21.31.
4-[4-(4-Benzhydryl-piperazin-1-yl)-6-(quinazolin-4-yloxy)-1,3,5-triazine-2-ylamino]-2-trifluoromethyl-benzonitrile (5o). Yield: 74%. m.p. 277–278°C (DMF). IR (KBr) cm−1: 3,289 (–NH), 2,224 (C≡N), 1,256 (C–O–C), 811 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.42 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.59 (s, 1H, N–CH–N of quinazoline ring), 8.13 (dd, J = 10.8, 4.9 Hz, 2H, quinazoline ring), 7.83 (t, J = 6.2 Hz, 1H, quinazoline ring), 7.60–7.27 (m, 14H, Ar–H), 4.70 (s, 1H, N–CH), 3.84 (br s, 4H, piperazine ring), 3.49 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 173.2 (C-6, s-triazine, C–N at piperazine linkage), 169.6 (C-2, s-triazine, C–NH at benzonitrile moiety), 165.4 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.8, 157.7, 157.0 (3C, Quinazoline heteroatom ring carbon atoms), 147.7–115.9 (24C, Ar. C), 104.8 (1C, C≡N), 97.7 (1C, –C–C≡N), 49.5, 45.1 (4C, piperazine ring carbon atoms). Anal. Calcd. for C36H28F3N9O: C, 65.55; H, 4.28; N, 19.11. Found: C, 65.76; H, 4.05; N, 18.90.
4-[4-{4-[(4-Chloro-phenyl)-phenyl-methyl]-piperazin-1-yl}-6-(quinazolin-4-yloxy)-1,3,5-triazine-2-ylamino]-2-trifluoromethyl-benzonitrile (5p). Yield: 76%. m.p. 269–271°C (DMF). IR (KBr) cm−1: 3,291 (–NH), 2,218 (C≡N), 1,257 (C–O–C), 811 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.66 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.51 (s, 1H, N–CH–N of quinazoline ring), 7.99 (dd, J = 11.3, 5.6 Hz, 2H, quinazoline ring), 7.75 (t, J = 7.1 Hz, 1H, quinazoline ring), 7.53-7.19 (m, 13H, Ar–H), 3.90 (s, 1H, N–CH), 3.79 (br s, 4H, piperazine ring), 3.46 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 172.8 (C-6, s-triazine, C–N at piperazine linkage), 169.0 (C-2, s-triazine, C–NH at benzonitrile moiety), 165.2 (C-4, s-triazine, C–O–C at quinazoline linkage), 157.1, 155.3, 155.1 (3C, Quinazoline heteroatom ring carbon atoms), 149.3–117.0 (24C, Ar. C), 105.1 (1C, C≡N), 97.7 (1C, –C–C≡N), 50.2, 44.9 (4C, piperazine ring carbon atoms). Anal. Calcd. for C36H27ClF3N9O: C, 62.29; H, 3.92; N, 18.16. Found: C, 61.97; H, 3.71; N, 18.30.
4-[4-[4-(2-Fluoro-phenyl)-piperazin-1-yl]-6-(quinazolin-4-yloxy)-1,3,5-triazine-2-ylamino]-2-trifluoromethyl-benzonitrile (5q). Yield: 77%. m.p. 275–276°C (THF). IR (KBr) cm−1: 3,266 (–NH), 2,221 (C≡N), 1,255 (C–O–C), 814 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.73 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.42 (s, 1H, N–CH–N of quinazoline ring), 8.02 (dd, J = 11.0, 7.3 Hz, 2H, quinazoline ring), 7.69 (t, J = 7.6 Hz, 1H, quinazoline ring), 7.51–7.34 (m, 10H, Ar–H), 6.90 (dd, J = 12.8, 6.4 Hz, 2H), 6.77–6.64 (m, 1H), 6.50 (dd, J = 12.7, 6.7 Hz, 1H), 3.84 (br s, 4H, piperazine ring), 3.50 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 172.2 (C-6, s-triazine, C–N at piperazine linkage), 168.7 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.4 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.8, 156.6, 155.2 (3C, Quinazoline heteroatom ring carbon atoms), 151.1 (1C, C–F), 150.1–117.8 (16C, Ar. C), 104.9 (1C, C≡N), 98.1 (1C, –C–C≡N), 50.0, 49.1 (4C, piperazine ring carbon atoms); 19F NMR (400 MHz,CDCl3) δ −124.1 (1F, s, 2-F), −67.8 (3F, s, –CF3). Anal. Calcd. for C29H21F4N9O: C, 59.28; H, 3.60; N, 21.46. Found: C, 59.12; H, 3.34; N, 21.31.
4-[4-[4-(4-Fluoro-phenyl)-piperazin-1-yl]-6-(quinazolin-4-yloxy)-1,3,5-triazine-2-ylamino]-2-trifluoromethyl-benzonitrile (5r). Yield: 75%. m.p. 292–293°C (THF). IR (KBr) cm−1: 3,274 (–NH), 2,222 (C≡N), 1,255 (C–O–C), 812 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.44 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.54 (s, 1H, N–CH–N of quinazoline ring), 7.98 (dd, J = 11.1, 7.5 Hz, 2H, quinazoline ring), 7.81 (t, J = 7.9 Hz, 1H, quinazoline ring), 7.56–7.33 (m, 10H, Ar–H), 7.02 (dd, J = 12.8, 6.9 Hz, 2H), 6.67–6.61 (m, 1H), 6.49 (dd, J = 12.1, 6.0 Hz, 1H), 3.44 (br s, 4H, piperazine ring), 3.85 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 173.3 (C-6, s-triazine, C–N at piperazine linkage), 168.8 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.5 (C-4, s-triazine, C–O–C at quinazoline linkage), 157.9, 156.6, 155.2 (3C, Quinazoline heteroatom ring carbon atoms), 153.3 (1C, C–F), 148.4–118.5 (16C, Ar. C), 105.4 (1C, C≡N), 97.1 (1C, –C–C≡N), 49.5, 45.0 (4C, piperazine ring carbon atoms); 19F NMR (400 MHz,CDCl3) δ −119.9 (1F, s, 4-F), −65.2 (3F, s, –CF3). Anal. Calcd. for C29H21F4N9O: C, 59.28; H, 3.60; N, 21.46. Found: C, 59.02; H, 3.79; N, 21.20.
4-{4-(Quinazolin-4-yloxy)-6-[4-(3-trifluoromethyl-phenyl)-piperazin-1-yl]-1,3,5-triazine-2-ylamino}-2-trifluoromethyl-benzonitrile (5s). Yield: 60%. m.p. >300°C (DMF). IR (KBr) cm−1: 3,289 (–NH), 2,220 (C≡N), 1,255 (C–O–C), 812 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.57 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.51 (s, 1H, N–CH–N of quinazoline ring), 8.21 (dd, J = 10.3, 7.2 Hz, 2H, quinazoline ring), 7.80 (t, J = 7.1 Hz, 1H, quinazoline ring), 7.57–7.25 (m, 15H, Ar–H), 3.52 (br s, 4H, piperazine ring), 3.89 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 172.7 (C-6, s-triazine, C–N at piperazine linkage), 168.6 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.8 (C-4, s-triazine, C–O–C at quinazoline linkage), 158.9, 157.3, 156.6 (3C, Quinazoline heteroatom ring carbon atoms), 151.1 (1C, C–F), 148.7–120.6 (17C, Ar. C including 2C-CF3 at 130.4, 130.0 & 2CF3 at 125.6, 124.9), 105.2 (1C, C≡N), 96.9 (1C, –C–C≡N), 49.8, 46.7 (4C, piperazine ring carbon atoms); 19F NMR (400 MHz,CDCl3) δ −66.9, −66.1 (6F, s, 2-CF 3); Anal. Calcd. for C30H21F6N9O: C, 56.52; H, 3.32; N, 19.77. Found: C, 56.39; H, 3.12; N, 19.96.
4-{4-(Quinazolin-4-yloxy)-6-[4-(2,3,4-trimethoxy-benzyl)-piperazin-1-yl]-1,3,5-triazine-2-ylamino}2-trifluoromethyl-benzonitrile (5t). Yield: 85%. m.p. 281–284°C (DMF). IR (KBr) cm−1: 3,269 (–NH), 2,219 (C≡N), 1,475(–CH2), 1,257 (C–O–C), 810 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 89.69 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.49 (s, 1H, N–CH–N of quinazoline ring), 8.16 (dd, J = 10.1, 6.9 Hz, 2H, quinazoline ring), 7.92 (t, J = 7.6 Hz, 1H, quinazoline ring), 7.45–7.29 (m, 6H, Ar–H), 6.91 (d, J = 7.4 Hz, 1H), 6.76 (d, J = 7.6 Hz, 1H), 3.78 (s, 9H, 3OCH 3), 3.79 (br s, 4H, piperazine ring), 3.48 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 171.9 (C-6, s-triazine, C–N at piperazine linkage), 168.7 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.8 (C-4, s-triazine, C–O–C at quinazoline linkage), 157.9, 156.6, 156.1 (3C, Quinazoline heteroatom ring carbon atoms), 149.3–115.9 (17C, Ar. C), 104.9 (1C, C≡N), 96.4 (1C, –C–C≡N), 61.6, 58.2, 57.1 (4C, 3OCH3 and –CH2), 52.0, 45.9 (4C, piperazine ring carbon atoms). Anal. Calcd. for C33H30F3N9O4: C, 58.84; H, 4.49; N, 18.71. Found: C, 58.98; H, 4.31; N, 18.82.
4-[4-[4-(4-Methoxy-phenyl)-piperazin-1-yl]-6-(quinazolin-4-yloxy)-1,3,5-triazine-2-ylamino]-2-trifluoromethyl-benzonitrile (5u). Yield: 88%. m.p. 270–272°C (THF). IR (KBr) cm−1: 3,285 (–NH), 2,224 (C≡N), 1,255 (C–O–C), 814 (s-triazine C–N str.). 1H NMR (400 MHz, DMSO-d6): δ = 9.71 (s, 1H, –NH, s-triazine to amino-benzonitrile linkage), 8.49 (s, 1H, N–CH–N of quinazoline ring), 8.09 (dd, J = 9.9, 6.1 Hz, 2H, quinazoline ring), 7.69 (t, J = 7.8 Hz, 1H, quinazoline ring), 7.56–7.29 (m, 6H, Ar–H), 7.11 (d, J = 7.7 Hz, 1H), 6.59 (d, J = 7.1 Hz, 1H), 4.03 (s, 3H, OCH 3), 3.89 (br s, 4H, piperazine ring), 3.44 (br s, 4H, piperazine ring). 13C NMR (100 MHz, DMSO-d6): δ = 172.4 (C-6, s-triazine, C–N at piperazine linkage), 168.5 (C-2, s-triazine, C–NH at benzonitrile moiety), 166.8 (C-4, s-triazine, C–O–C at quinazoline linkage), 159.0, 158.1, 157.7 (3C, Quinazoline heteroatom ring carbon atoms), 148.7–118.5 (17C, Ar. C), 105.0 (1C, C≡N), 99.0 (1C, –C–C≡N), 59.2 (1C, –OCH3), 50.9, 46.3 (4C, piperazine ring carbon atoms). Anal. Calcd. for C30H24F3N9O2: C, 60.10; H, 4.03; N, 21.03. Found: C, 59.93; H, 4.21; N, 20.82.
Methods of in vitro evaluation of biological activities
The synthesized s-triazinyl derivatives 5a–5u were examined for their antimicrobial activities against several bacteria (Staphylococcus aureus MTCC 96, Bacillus cereus MTCC 619, Escherichia coli MTCC 739, Pseudomonas aeruginosa MTCC 741, Klebsiella pneumoniae MTCC 109, Salmonella typhi MTCC 733, Proteus vulgaris MTCC 1771, and Shigella Flexneria MTCC 1457) and fungi (Aspergillus niger MTCC 282, Aspergillus fumigatus MTCC 343, Aspergillus clavatus MTCC 1323, and Candida albicans MTCC 183) species using paper disk-diffusion technique (Gillespie, 1994), and MIC of the compound was determined by agar streak dilution method (Hawkey and Lewis, 1994). The preliminary antimycobacterial assessment for the final synthesized compounds was carried out using BACTEC MGIT method [Isenberg, 1992], and the secondary antimycobacterial screening for test compounds was obtained for M. tuberculosis H37 Rv, by means of L. J. (Lowenstein and Jensen) MIC method [Anargyros et al., 1990]. Detailed procedures of pharmacologic evaluations were described earlier (Patel et al., 2011a, b).
Conclusion
In this article, we have presented the initial efforts made toward the discovery of novel, potentially active 2-(4-cyano-3-trifluoromethylphenyl amino)-4-(4-quinazolinyloxy)-6-piperazinyl(piperidinyl)-s-triazines. Owing to the presence of three pharmacologically active nuclei, viz., s-triazine, quinazoline, and piperazines or piperidines, in one single molecule, compounds have potent antimicrobial and antituberculosis effect. From the bioassay it is clear that the introduction of appropriate substituent on the s-triazine ring would lead to the more active antimicrobial derivatives. It can be stated that the variation of antimicrobial activity may be associated with the nature of tested microorganisms and is due to the chemical structure of the tested compounds. In the present study, higher potency has been observed with the final compounds bearing piperazine bases with electron-withdrawing groups like chlorine and fluorine atom(s) and electron-releasing methyl and methoxy functional group(s). These privileged structures with their enhanced bioactivities represent an ideal source of core scaffolds for the design of molecules with an ability to target various pathogenic microbes for further drug discovery process.
References
Anargyros P, Astill DS, Lim IS (1990) Comparison of improved BACTEC and Lowenstein-Jensen media for culture of mycobacteria from clinical specimens. J Clin Microbiol 28:1288–1291
Baliani A, Bueno GJ, Stewart ML, Yardley V, Brun R, Barrett MP, Gilbert IH (2005) Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J Med Chem 48:5570–5579
Chikhalia KH, Patel MJ (2009) Design, synthesis and evaluation of some 1,3,5-triazinyl urea and thiourea derivatives as antimicrobial agents. J Enzym Inhib Med Chem 24:960–966
Gillespie SH (1994) Medical microbiology—illustrated. Butterworth Heinemann Ltd., United Kingdom
Hawkey PM, Lewis DA (1994) Medical bacteriology—a practical approach. Oxford University Press, United Kingdom
Isenberg HD (1992) Clinical microbiology procedures handbook, vol 1. American Society for Microbiology, Washington, DC
Karminski W, Kulicka J, Miernik J (1983) The synthesis of some quinazoline derivatives and their biological properties. J Environ Sci Health B Pestic Food Contam Agric Wastes 18:599–610
Kerns RJ, Rybak MJ, Kaatz GW, Vaka F, Cha R, Grucz RG, Diwadkar VU, Ward TD (2003) Piperazinyl-linked fluoroquinolone dimers possessing potent antibacterial activity against drug-resistant strains of Staphylococcus aureus. Bioorg Med Chem Lett 13:1745–1749
Kumar CS, Vinaya K, Chandra JN, Thimmegowda NR, Prasad SB, Sadashiva CT, Rangappa KS (2008) Synthesis and antimicrobial studies of novel 1-benzhydryl-piperazine sulfonamide and carboxamide derivatives. J Enzyme Inhib Med Chem 23:462–469
Mahajan DH, Pannecouque C, Erik DC, Chikhalia KH (2009) Synthesis and studies of new 2-(coumarin-4-yloxy)-4,6-(substituted)-s-triazine derivatives as potential anti-HIV agents. Arch Pharm 342:281–290
Menicagli R, Samaritani S, Signore G, Vaglini F, Dalla Via L (2004) In vitro cytotoxic activities of 2-alkyl-4,6-diheteroalkyl-1,3,5-triazines: # new molecules in anti cancer research. J Med Chem 47:4649–4652
Nathan C (2004) Antibiotics at the crossroads. Nature 431:899–902
Nosova EV, Lipunova GN, Charushin VN (2009) Fluorine-containing quinazolines and their oxa and thia analogues: synthesis and biological activities. Russ Chem Rev 78:387–406
Patel DH, Chikhalia KH, Shah NK, Patel DP, Kaswala PB, Buha VM (2010a) Synthesis and antimicrobial studies of s-triazine based heterocycles. J Enzym Inhib Med Chem 25:121–125
Patel RV, Kumari P, Chikhalia KH (2010b) Novel s-triazinyl piperazines: design, synthesis, characterization and anti-microbial activity. Arch Appl Sci Res 2:232–240
Patel RV, Kumari P, Chikhalia KH (2011a) Fluorinated s-triazinyl piperazines as antimicrobial agents. Z Naturforsch C J Biosci 66:341–345
Patel RV, Kumari P, Rajani DP, Chikhalia KH (2011) A new class of 2-(4-cyanophenyl amino)-4-(6-bromo-4-quinolinyloxy)-6-piperazinyl (piperidinyl)-1,3,5-triazine analogues with antimicrobial/antimycobacterial activity. J Enzyme Inhib Med Chem, in press. doi:10.3109/14756366.2011.592491
Patel RV, Kumari P, Rajani DP, Chikhalia KH (2011c) Synthesis and studies of novel 2-(4-cyano-3-trifluoromethylphenyl amino)-4-(quinoline-4-yloxy)-6-(piperazinyl/piperidinyl)-s-triazines as potential antimicrobial, antimycobacterial and anticancer agents. Eur J Med Chem 46:4354–4365
Raviglione MC (2003) The TB epidemic from 1992 to 2002. Tuberculosis (Edinb) 83:4–14
WHO report (2011) Global tuberculosis control. Available at http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf
Sergio M, Davide P, Paolo C, Nicoletta B, Diego M (2008) A combinatorial approach to 2,4,6-trisubstituted triazines with potent antimalarial activity: combining conventional synthesis and microwave-assistance. ChemMedChem 3:873–876
Srinivas K, Srinivas U, Bhanuprakash K, Harakishore K, Murthy USN, Jayathirtha RV (2006) Synthesis and antibacterial activity of various substituted s-triazines. Eur J Med Chem 41:1240–1246
Sunduru N, Gupta L, Chaturvedi V, Dwivedi R, Sinha S, Chauhan PMS (2010) Discovery of new 1,3,5-triazines scaffolds with potent activity against Mycobacterium tuberculosis H37Rv. Eur J Med Chem 45:3335–3345
Tran TP, Ellsworth EL, Stier MA, Domagala JM, Hollis Showalter HD, Gracheck SJ, Shapiro MA, Joannides TE, Singh R (2004) Synthesis and structural–activity relationships of 3-hydroxyquinazoline-2,4-dione antibacterial agents. Bioorg Med Chem Lett 14:4405–4409
Tsizin YS, Karpova NB, Lukyanov AV, Rudzum EA, Kulikova DA, Radkevich TP (1973) Synthesis and antimicrobial activity of N-substituted 2-ethyl-4-amino-6-hydroxyquinazolines. Pharm Chem J 7:16–19
Upadhayaya RS, Sinha N, Jain S, Kishore N, Chandra R, Arora SK (2004) Optically active antifungal azoles: synthesis and antifungal activity of (2R, 3S)-2-(2,4-difluorophenyl)-3-(5-{2-[4-aryl-piperazin-1-yl]-ethyl}-tetrazol-2-yl/1-yl)-1-[1,2,4]-triazol-1-yl-butan-2-ol. Bioorg Med Chem 12:2225–2238
Xiong YZ, Chen FE, Balzarini J, De Clercq E, Pannecouque C (2008) Non-nucleoside HIV-1 reverse transcriptase inhibitors. Part 11: structural modulations of diaryltriazines with potent anti-HIV activity. Eur J Med Chem 43:1230–1236
Xiong YZ, Chen FE, Balzarini J, De Clercq E (2009) Non-nucleoside HIV-1 reverse transcriptase inhibitors: part 13: synthesis of fluorine-containing diaryltriazine derivative for in vitro anti-HIV evaluation against wild-type strain. Chem Biodivers 6:561–568
Zhou C, Min J, Zhigang L, Anne Y, Heather D, Tian G, Young-Tae C, Neville RK (2008) Synthesis and biological evaluation of novel 1,3,5-triazine derivatives as antimicrobial agents. Bioorg Med Chem Lett 18:308–1311
Acknowledgments
The authors are thankful to the faculty of the Applied Chemistry Department of S. V. National Institute of Technology, Surat for providing the scholarship, encouragement, and facilities. The authors wish to offer their deep gratitude to the concerned authorities of the Microcare Laboratory, Surat, India for assistance in carrying out the biological screening, and the Centre of Excellence, Vapi, India, for their assistance during 1H NMR, 13C NMR, and 19F NMR analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, R.V., Kumari, P., Rajani, D.P. et al. Discovery of 2-(4-cyano-3-trifluoromethylphenyl amino)-4-(4-quinazolinyloxy)-6-piperazinyl(piperidinyl)-s-triazines as potential antibacterial agents. Med Chem Res 21, 4177–4192 (2012). https://doi.org/10.1007/s00044-011-9950-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9950-4