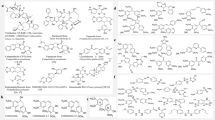
Abstract
This study is based on the synthesis of a series of combretastatin analogs with different substitutions on one aryl moiety and a carboxylic group in connecting chain. Cis-configuration with respect to aryl groups was established by X-ray crystal analysis. All the synthesized compounds were evaluated for anticancer activity against a panel of cell lines. Six compounds 1a, 1b, 1c, 1k, 1n, and 1p showed marked anticancer activity against human colon (colo-205), lung (A549), ovary (IGROV-1), prostrate (PC-3), CNS (SF-295), leukemia (THP-1), and breast (MCF-7) cell lines. Out of these, 1b showed remarkable inhibitory activity comparable to paclitaxel against lung cancer cell line with IC50 3.9 μM. Importance of carboxylic group in the synthesized compounds was studied by flexible docking study of 1b which showed the importance of carboxylic group interactions with colchicine-binding site of αβ-tubulin.
Graphical Abstract
A series of combretastatin analogs have been synthesized by condensation of phenyl acetic acid and different substituted aldehydes. All the synthesized compounds were evaluated for anticancer activity against a panel of cell lines. Six compounds 1a, 1b, 1c, 1k, 1n, and 1p showed better anticancer activity against human colon (colo-205), lung (A549), ovary (IGROV-1), prostrate (PC-3), CNS (SF-295), leukemia (THP-1), and breast (MCF-7) cell lines. Out of these, 1b showed marked inhibitory activity against lung cancer cell line with IC50 3.9 μM.





Similar content being viewed by others
References
Bellina F, Cauteruccio S, Monti S, Rossi R (2006) Novel imidazole-based combretastatin A-4 analogues: evaluation of their in vitro antitumor activity and molecular modelling study of their binding to the colchicine site of tubulin. Bioorg Med Chem Lett 16:5757–5762
Brown RT, Fox BW, Hadfield JA, McGown AT, Mayalarp SP, Pettit GR, Woods JA (1995) Synthesis of water-soluble sugar derivatives of combretastatin A-4. J Chem Soc Perkin Trans 1:577–582
ChemDraw Ultra 6.0 and Chem3D Ultra (2000) Cambridge Soft Corporation, Cambridge
Cushman MS, Layfayette W, Hamel E, Bethesda (1995) Stilbene derivatives as anticancer agents. United States Patent. Patent no 5,430,062
Ducki S, Mackenzie G, Greedy B, Armitage S, Chabert JF, Bennett E, Nettles J, Snyder JP, Lawrence NJ (2009) Combretastatin-like chalcones as inhibitors of microtubule polymerisation. Part 2: Structure-based discovery of alpha-aryl chalcones. Bioorg Med Chem 17:7711–7722
Furst R, Zupko I, Berenyi A, Ecker GF, Rinner U (2009) Synthesis and antitumor-evaluation of cyclopropyl-containing combretastatin analogs. Bioorg Med Chem Lett 19:6948–6951
GOLD (2009) Evaluation version 4.0.1. Cambridge Crystallographic Data Centre, Cambridge
Hadfield JA, Ducki S, Hirst N, McGown AT (2003) Tubulin and microtubules as targets for anticancer drugs. Prog Cell Cycle Res 5:309–325
Kaffy J, Pontikis R, Carrz D, Croisy A, Monneret C, Florent JC (2006) Isoxazole-type derivatives related to combretastatin A-4, synthesis and biological evaluation. Bioorg Med Chem 14:4067–4077
Kong Y, Grembecka J, Edler MC, Hamel E, Mooberry SL, Sabat M, Rieger J, Brown ML (2005) Structure-based discovery of a boronic acid bioisostere of combretastatin A-4. Chem Biol 12:1007–1014
Lee L, Davis R, Vanderham J, Hills P, Mackay H, Brown T, Mooberry SL, Lee M (2008) 1,2,3,4-Tetrahydro-2-thioxopyrimidine analogs of combretastatin-A4. Eur J Med Chem 43:2011–2015
Liou JP, Chang YL, Kuo FM, Chang CW, Tseng HY, Wang CC, Yang YN, Chang JY, Lee SJ, Hsieh HP (2004) Concise synthesis and structure–activity relationship of combretastatin A-4 analogues, 1-aroylindoles, as novel classes of potent antitubulin agents. J Med Chem 47:4247–4257
Liou JP, Wu ZY, Kuo CC, Chang CY, Lu PY, Chen CM, Hsieh HP, Chang JY (2008) Discovery of 4-amino and 4-hydroxy-1-aroylindoles as potent tubulin polymerization inhibitors. J Med Chem 51:4351–4355
McGown AT, Fox BW (1989) Structural and biochemical comparison of the anti-mitotic agents colchicine, combretastatin A4 and amphethinile. Anticancer Drug Des 3:249–254
Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–766
Nandy P, Banerjee S, Gao H, Hui MB, Lien EJ (1991) Quantitative structure–activity relationship analysis of combretastatins: a class of novel antimitotic agents. Pharm Res 8:776–781
Ouyang X, Piatnitski EL, Pattaropong V, Chen X, He HY, Kiselyov AS, Valankar A, Kwakami J, Labelle M, Smith L, Lohman J, Lee SP, Malikzay A, Fleming J, Gerlak J, Wang Y, Rosler RL, Zhou K, Mitelman S, Camara M, Surguladze D, Boody JF, Tuma MC (2006) Oxadiazole derivatives as a novel class of antimitotic agents: synthesis, inhibition of tubulin polymerization and activity in tumor cell lines. Bioorg Med Chem Lett 16:1191–1196
Pettit GR, Singh SB, Niven ML, Hamel E, Schmit JM (1987) Isolation, structure and synthesis of combretastatin A-1 and B-, potent new inhibitors of microtubule assembly, derived from Combretum caffrum. J Nat Prod 50:119–120
Ravelli RB, Gigant B, Curmi PA, Jourdain I, Lachkar S, Sobel A, Knossow M (2004) Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 428:198–202
Shewach DS, Kuchta RD (2009) Introduction to cancer chemotherapeutics. Chem Rev 109:2859–2861
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Stokvis E, Nan-Offeringa L, Ouwehand M, Tibben MM, Rosing H, Schnaar Y, Grigat M, Romeis P, Schellens JTM, Beijnen JH (2004) Quantitative analysis of D-24851, a novel anticancer agent, in human plasma and urine by liquid chromatography coupled with tandem mass spectrometry. Cancer Treat Rev 18:1465–1471
The Merck Index (2006) Fourteenth edition, Organic Name Reactions-324
Tron GC, Pirali T, Sorba G, Pagliai F, Busacca S, Genazzani AA (2006) Medicinal chemistry of combretastatin A4: present and future directions. J Med Chem 49:3033–3044
Vincent L, Kermani P, Young LM, Cheng J, Zhang F, Shido K, Lam G, Bompais VH, Zhu Z, Hicklin DJ, Bohlen P, Chaplin DJ, May C, Raffi SJ (2005) Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signalling. Clin Investig 115:2992
Woods JA, Hadfield JA, Pettit GR, Fox BW, McGown AT (1995) The interaction with tubulin of a series of stilbenes based on combretastatin A-4. Br J Cancer 71:705–711
Acknowledgments
The authors are grateful to ISF College of Pharmacy, Moga for providing research facilities; IIIM, Jammu for spectral analysis as well pharmacological activity and to Prof. Rajnikant and Dr. Vivek Gupta, University of Jammu, Jammu Tawi, for X-ray crystal analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kumar, S., Sapra, S., Kumar, R. et al. Synthesis of combretastatin analogs: evaluation of in vitro anticancer activity and molecular docking studies. Med Chem Res 21, 3720–3729 (2012). https://doi.org/10.1007/s00044-011-9887-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9887-7