Abstract
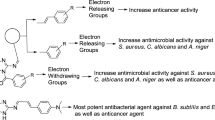
The benzo[b][1,4]oxazin-3(4H)-one derivatives, 1a–p, carrying F, Br, and Cl on the benzene ring, or benzyl, cyclohexyl, n-hexyl, and tetrafuryl methylene groups attached to nitrogen atom were synthesized via Smiles rearrangement and assayed in vitro for their antimicrobial activity against Gram-positive, Gram-negative bacteria, and fungi. The antimicrobial activity of the benzo[b][1,4]oxazin-3(4H)-ones showed, on the whole, potency toward all the tested Gram-positive and Gram-negative microorganism (MIC ranging from 16 to 64 μg/ml), whereas weak effectiveness was exhibited against fungi. Data obtained suggest that fluorine atom in the compounds, 1c, 1f, 1i plays an important role in enhancing the antimicrobial properties of this class of compounds. These observations provide some predictions to design further antimicrobial active compounds prior to their synthesis according to molecular modeling studies.




Similar content being viewed by others
References
Anderluh M, Cesar J, Štefanič P, Kikelj D, Janeš D, Murn J, Nadrah K, Tominc M, Addicks E, Giannis A, Stegnar M, Dolenc MS (2005) Design and synthesis of novel platelet fibrinogen receptor antagonists with 2H–1,4-benzoxazine-3(4H)-one scaffold. A systematic study. Eur J Med Chem 40(1):25–49
Buckman BO, Mohan R, Koovakkat S, Liang A, Trinh L, Morrissey MM (1998) Design, synthesis, and biological activity of novel purine and bicyclic pyrimidine factor Xa inhibitors. Bioorg Med Chem Lett 8(16):2235–2240
Caliendo G, Perissutti E, Santagada V, Fiorino F, Severino B, d’Emmanuele di Villa Bianca R, Lippolis L, Pinto A, Sorrentinio R (2002) Synthesis and vasorelaxant activity of new 1,4-benzoxazine derivatives potassium channel openers. Bioorg Med Chem 10(8):2663–2669
Cho S-D, Park Y-D, Kim J-J, Lee S-G, Ma C, Song S-Y, Joo W-H, Falck JR, Shiro M, Yoon Y-J, Shin D-S, Yoon Y-J (2003) A one-pot synthesis of pyrido[2,3-b][1,4]oxazin-2-ones. J Org Chem 68(20):7918–7920
Cho S-D, Park Y-D, Kim J-J, Joo W-H, Shiro M, Esser L, Falck JR, Ahn C, Yoon Y-J, Shin D-S (2004) An efficient synthesis of [1,4]pyridazinooxazine[3,4-a] tetrahydro isoquinolines. Tetrahedron 60(17):3763–3773
Christie RM, Agyako CK, Mitchell K (1995) An investigation of the electronic spectral properties of the merocyanines derived from photochromic spiroindolinonaphth[2,1-b][1,4]oxazines. Dyes Pigments 29(3):241–250
Ericcsson HM, Sherris JC (1971) Antibiotic sensitivity testing: report of an international collaborative study. Acta Pathol Microbiol Scand B 217:1–90
Fringuelli R, Pietrella D, Schiaffella F, Guarraci A, Perito S, Bistoni F, Vecchiarelli A (2002) Anti-Candida albicans properties of novel benzoxazine analogues. Bioorg Med Chem 10(6):1681–1686
Huang M-Z, Huang K-L, Ren Y-G, Lei M-X, Huang L, Hou Z-K, Liu A-P, Qu X-M (2005) Synthesis and herbicidal activity of 2-(7-fluoro-3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-6-yl)isoindoline-1,3-diones. J Agric Food Chem 53(20):7908
Kajino M, Shibouta Y, Nishikawa K, Meguro K (1991) Synthesis and biological activities of new 2-substituted 1,4-benzoxazine derivatives. Chem Pharm Bull 39(11):2896–2905
Katsura Y, Nishino S, Takasugi H (1991) Studies on antiulcer drugs. I. Synthesis and antiulcer activities of imidazo[1,2-alpha]pyridinyl-2-oxobenzoxazolidines-3-oxo-2H–1,4-benzoxazines and related compounds. Chem Pharm Bull 39(11):2937–2943
Lanni TB Jr, Greene KL, Kolz CN, Para KS, Visnick M, Mobley JL, Dudley DT, Baginski TJ, Liimatta MB (2007) Design and synthesis of phenethyl benzo[1,4]oxazine-3-ones as potent inhibitors of PI3Kinaseγ. Bioorg Med Chem Lett 17(3):756–760
Ma C, Cho S-D, Falck JR, Shin D-S (2004) Synthetic studies on isoquinoline derivatives with multidrug resistance (MDR) modulating activity. Heterocycles 63(1):75–85
Macchiarulo A, Costantino G, Fringuelli D, Vecchiarelli A, Schiaffella F, Fringuelli R (2002) 1,4-Benzothiazine and 1,4-benzoxazine imidazole derivatives with antifungal activity: a docking study. Bioorg Med Chem 10(11):3415–3423
Muroi H, Nihei K, Tsujimoto K, Kubo I (2004) Synergistic effects of anacardic acids and methicillin against methicillin resistant Staphylococcus aureus. Bioorg Med Chem 12(3):583–587
Nair MG, Salter OC, Kisliuk RL, Gaumont Y, North G (1983) Folate analogs. 22. Synthesis and biological evaluation of two analogs of dihydrofolic acid possessing a 7,8-dihydro-8-oxapterin ring system. J Med Chem 26(8):1164–1168
Roberts MC (2004) Distribution of macrolide, lincosamide, streptogramin, ketolide and oxazolidinone (MLSKO) resistance genes in gram-negative bacteria. Curr. Drug Targets Infect Disord 4(3):207–215
Shin D-S, Park JK (2007) Characteristics of the intermediates in the cyclization reactions of heterocycle-fused [1,4]oxazine derivatives: stepwise versus concerted. Bull Korean Chem Soc 28(12):2219–2225
Smid P, Coolen HKAC, Keizer HG, van Hes R, de Moes J-P, den Hartog AP, Stork B, Plekkenpol RH, Niemann LC, Stroomer CNJ, Tulp MTM, van Stuivenberg HH, McCreary AC, Hesselink MB, Herremans AHJ, Kruse CG (2005) Synthesis, structure-activity relationships, and biological properties of 1-heteroaryl-4-[ω-(1H-indol-3-yl) alkyl]piperazines, novel potential antipsychotics combining potent dopamine D2 receptor antagonism with potent serotonin reuptake inhibition. J Med Chem 48(22):6855–6869
Stalons DR, Thornsberry C (1975) Broth dilution for determining the antibiotic susceptibility of anaerobic bacteria. Antimicrob Agents Chemother 7(1):15–21
Sun XD, Fan MG, Meng XJ, Knobbe ET (1997) Acidichromic effects in spiro (1,3,3-trimethylindolo-2,3′-naphth[1,2-b]-1,4-oxazine) a photochromic compound I. Absorption characteristics. J Photochem Photobiol A 102(2–3):213–216
Tenover FC, McDonald LC (2005) Vancomycin-resistant staphylococci and enterococci: epidemiology and control. Curr Opin Infect Dis 18(4):300–305
Wahidulla S, Bhattacharjee JJ (2001) Benzoxazinoids from Acanthus illicifolius. J Indian Inst Sci 81:485–490
Zuo H, Meng L, Ghate M, Hwang K-H, Cho Y-K, Chandrasekhar S, Reddy CR, Shin D-S (2008) Microwave-assisted one-pot synthesis of benzon[b][1,4] oxazin-3(4H)-ones via Smiles rearrangement. Tetrahedron Lett 49(23):3827–3830
Acknowledgements
The authors acknowledge the financial support of the grants from Natural Science Foundation Project of CQ CSTC, 2009BB5007 and National Research Foundation of Korea Grant funded by the Korean Government (2009-0073064).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fang, L., Zuo, H., Li, ZB. et al. Synthesis of benzo[b][1,4]oxazin-3(4H)-ones via smiles rearrangement for antimicrobial activity. Med Chem Res 20, 670–677 (2011). https://doi.org/10.1007/s00044-010-9360-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9360-z