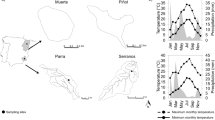
Abstract
Herbivory has been shown to play a strong role in controlling primary productivity in tidal salt marshes, but little work has been conducted in low salinity marshes. We measured aboveground plant biomass and nutrient response to insect exclusion along a salinity gradient from tidal freshwater to oligohaline marshes. We expected higher biomass in plants protected from herbivory and more so in the higher salinity marshes where tissue quality was anticipated to improve. Among three marshes along the salinity gradient, aboveground biomass within experimental plots did not vary across control and insect exclosure treatments. Overall tissue quality did not increase with increasing salinity, thus we did not find increasing grazing pressure along that gradient. Tissue N-content in Zizania aquatica (a low-salt tolerant, C3, annual grass) was significantly higher (p < 0.05) in plants within the insect exclosures, and it is the only species of those tested to demonstrate this effect. The lower tissue N-content in controls of this species could be either a response to grazing pressure that disrupts the ability of the plant to develop amino acids or a loss of N through guttation. We found that increasing levels of salt may have little effect on biomass in these marshes, but nutrient dynamics may shift as species like Zizania adjust the pool of tissue N-content.




Similar content being viewed by others
References
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol) 57:289–300
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29:1165–1188
Bertness MD, Shumway SW (1993) Competition and facilitation in marsh plants. Am Nat 142:718–724
Bertness MD, Wise C, Ellison A (1987) Consumer pressure and seed set in New England marsh perennials. Oecologia 71:191–200
Bertness MD, Crain CM, Holdredge C, Sala N (2008) Eutrophication and consumer control of New England salt marsh primary productivity. Conserv Biol 22:131–139
Bradley PM, Morris JT (1990) Influences of oxygen and sulfide concentration on nitrogen uptake kinetics in Spartina alterniflora. Ecology 71:282–287
Cardinale BJ, Matulich KL, Hooper DU, Byrnes JE, Duffy JE, Gamfelt L, Balvanera P, O’Connor MI, Gonzales A (2011) The functional role of producer diversity in ecosystems. Am J Bot 98:1–21
Chambers RM, Fourqurean JM (1991) Alternative criteria for assessing nutrient limitation of a wetland macrophyte (Peltandra virginica (L.) Kunth). Aquat Bot 40:305–320
Couture JJ, Servi JS, Lindroth RL (2010) Increased nitrogen availability influenced predator-prey interactions by altering host-plant quality. Chemoecology 20:277–284
Crain CM (2008) Interactions between marsh plant species vary in direction and strength depending on environmental and consumer context. J Ecol 96:166–173
Daehler CC, Strong DR (1995) Impact of high herbivore densities on introduced smooth cordgrass, Spartina alterniflora, invading San Francisco Bay, California. Estuaries 18:409–417
Daehler CC, Strong DR (1996) Reduced herbivore resistance in introduced smooth cordgrass (Spartina alterniflora) after a century herbivore-free growth. Oecologia 110:99–108
Davies SD (2004) Vegetation dynamics of a tidal freshwater marsh: Long-term and inter-annual variability and their relationship to salinity. MS Thesis, The College of William and Mary School of Marine Science. Gloucester Point, VA (USA). https://doi.org/10.25773/v5-3ghv-5c27
Eskelinen A, Harrison S, Tuomi M (2012) Plant traits mediate consumer and nutrient control on plant community productivity and diversity. Ecology 93:2705–2718
Finke DL, Denno RF (2005) Predator diversity and the functioning of ecosystems: the role of intraguild predation in dampening trophic cascades. Ecol Lett 8:1299–1306
Goatley JL, Lewis RW (1966) Composition of guttation fluid from rye, wheat, and barley seedlings. Plant Physiol 41:373–375
Haines BL, Dunn EL (1976) Growth and resource allocation responses of Spartina alterniflora to three levels of NH4–N, Fe, and NaCl in solution culture. Bot Gaz 137:224–230
Howes BL, Howarth RW, Teal JM, Valiela I (1981) Oxidation–reduction potentials in a salt marsh: Spatial patterns and interactions with primary production. Limnol Oceanogr 26:350–360
Leriche H, LeRoux X, Gignoux J, Tuzet A, Fritz H, Abbadie L, Loreau M (2001) Which functional processes control the short-term effect of grazing on net primary production in grasslands? Oecologia 129:114–124
Levine JM, Hacker SD, Harley CGC, Bertness MD (1998) Nitrogen effects on an interaction chain in a salt marsh community. Oecologia 117:266–272
Mendelssohn IA, Mckee KL, Patrick WH, jr (1981) Oxygen deficiency in Spartina alterniflora roots: metabolic adaptation to anoxia. Science 214:439–441
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Neubauer SC, Anderson IC (2003) Transport of dissolved inorganic carbon from a tidal freshwater marsh to the York River estuary. Limnol Oceanogr 48:299–307
NOAA: National Oceanic and Atmospheric Administration, Office of Ocean and Coastal Resource Management, National Estuarine Research Reserve System-wide Monitoring Program (2012) Centralized Data Management Office, Baruch Marine Field Lab, University of South Carolina. http://cdmo.baruch.sc.edu
Osgood DT, Zieman JC (1993) Factors controlling aboveground Spartina alterniflora (smooth cordgrass) tissue element composition and production in different-age barrier island marshes. Estuaries 16:815–826
Parys K, Johnson SJ (2011) Collecting insects associated with wetland vegetation: an improved design for a floating pitfall trap. Coleopt Bull 65:341–344
Pennings SC, Carefoot TH, Siska EL, Chase ME, Page TA (1998) Feeding preferences of a generalist salt marsh crab: relative importance of multiple plant traits. Ecology 79:1968–1979
Pennings SC, Ho CK, Salgado CS, Więski K, Davé N, Kunza AE, Wason EL (2009) Latitudinal variation in herbivore pressure in Atlantic Coast salt marshes. Ecology 90:183–195
Perry JE, Atkinson RB (2009) York River tidal marshes. J Coastal Res 57(sp1):40–49
Perry JE, Hershner CH (1999) Temporal changes in the vegetation pattern in a tidal freshwater marsh. Wetlands 19:90–99
Pierfelice KN, Lockaby BD, Krauss KW, Conner WH, Noe GB, Ricker MC (2015) Salinity influences on aboveground and belowground net primary productivity in tidal wetlands. J Hydrol Eng 22:1. https://doi.org/10.1061/(ASCE)HE.1943-5584.0001223
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reay WG, Moore KA (2009) Introduction to the Chesapeake Bay National Estuarine Research Reserve in Virginia. J Coastal Res 57(sp1):1–9
Rypstra AL, Buddle CM (2013) Spider silk reduces insect herbivory. Biol Let. https://doi.org/10.1098/rsbl.2012.0948
Schmitz OJ, Krivan V, Ovadia O (2004) Trophic cascades: the primacy of trait-mediated indirect interactions. Ecol Lett 7:153–163
Silliman BR, Zieman JC (2001) Top-down control of Spartina alterniflora production by periwinkle grazing in a Virginia salt marsh. Ecology 82(10):2830–2845
Sullivan MJ, Daiber FC (1974) Response in production of cordgrass, Spartina alterniflora, to inorganic nitrogen and phosphorus fertilizer. Chesapeake Sci 15:121–123
Sutter LA (2014) Effects of Saltwater Intrusion on Vegetation Dynamics and Nutrient Pools in Low-Salinity Tidal Marshes, Pamunkey River (Virginia, USA). Ph.D. Dissertation, The College of William and Mary School of Marine Science. Gloucester Point, VA (USA). https://doi.org/10.25773/v5-db2g-qt58
Sutter LA, Perry JE, Chambers RM (2014) Tidal freshwater marsh plant responses to low level salinity increases. Wetlands 34:167–175
Sutter LA, Chambers RM, Perry JE (2015) Seawater intrusion mediates species transition in low salinity, tidal marsh vegetation. Aquat Bot 122:32–39
Weakley AS, Ludwig JC, Townsend JF (2012) Flora of Virginia. BRIT Press, Fort Worth (TX), 1554p
Acknowledgements
We thank Scott Lerberg and Jim Goins who surveyed for insects and/or supported field work. We also thank Scott Neubauer, J. Emmett Duffy, Carl H. Hobbs, III, Daniel Markewitz, Mac Callaham and anonymous reviewers for early reviews of this manuscript. This paper was developed under STAR Fellowship Assistance Agreement no. FP-91736901 awarded by the U.S. Environmental Protection Agency (EPA). It has not been formally reviewed by EPA. The views expressed in this paper are solely those of Lori A. Sutter and her co-authors, and EPA does not endorse any products or commercial services mentioned in this paper. This paper is Contribution No. 3799 of the Virginia Institute of Marine Science, William & Mary.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sutter, L.A., Chambers, R.M., Karp, M. et al. A test of top-down control on plant production and nutrient quality in low-salinity tidal marshes. Aquat Sci 81, 16 (2019). https://doi.org/10.1007/s00027-018-0616-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-018-0616-x