Abstract
The Bone Morphogenetic Protein (BMP) signaling pathway has established roles in early embryonic morphogenesis, particularly in the epiblast. More recently, however, it has also been implicated in development of extraembryonic lineages, including trophectoderm (TE), in both mouse and human. In this review, we will provide an overview of this signaling pathway, with a focus on BMP4, and its role in emergence and development of TE in both early mouse and human embryogenesis. Subsequently, we will build on these in vivo data and discuss the utility of BMP4-based protocols for in vitro conversion of primed vs. naïve pluripotent stem cells (PSC) into trophoblast, and specifically into trophoblast stem cells (TSC). PSC-derived TSC could provide an abundant, reproducible, and ethically acceptable source of cells for modeling placental development.




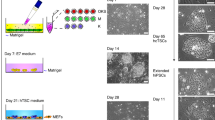
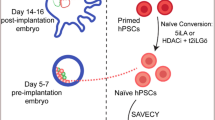
Modified from Fig. 3A in [131]. B GATA2, GATA3, TFAP2C, and BMP4 gene expressions during reprogramming to naïve and primed human pluripotency prepared from interactive online tool (http://hrpi.ddnetbio.com/) by [131]. Gene expressions are shown in log-transformed log2 (FPKM + 1) and days (D) or passage numbers (P) from the OSKM transduction. Black, blue, and orange lines represent gene expression in fibroblast, naïve (t2iLGoY), and primed medium, respectively
Similar content being viewed by others
Availability of data and material
Not applicable.
References
Sieber C, Kopf J, Hiepen C, Knaus P (2009) Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev 20:343–355. https://doi.org/10.1016/J.CYTOGFR.2009.10.007
Bragdon B, Moseychuk O, Saldanha S et al (2011) Bone Morphogenetic Proteins: a critical review. Cell Signal 23:609–620. https://doi.org/10.1016/J.CELLSIG.2010.10.003
Yadin D, Knaus P, Mueller TD (2016) Structural insights into BMP receptors: specificity, activation and inhibition. Cytokine Growth Factor Rev 27:13–34
García de Vinuesa A, Abdelilah-Seyfried S, Knaus P et al (2016) BMP signaling in vascular biology and dysfunction. Cytokine Growth Factor Rev 27:65–79. https://doi.org/10.1016/J.CYTOGFR.2015.12.005
Ross S, Hill CS (2008) How the Smads regulate transcription. Int J Biochem Cell Biol 40:383–408. https://doi.org/10.1016/J.BIOCEL.2007.09.006
Hussein SM, Duff EK, Sirard C (2003) Smad4 and β-catenin Co-activators functionally interact with lymphoid-enhancing factor to regulate graded expression of Msx2 *. J Biol Chem 278:48805–48814. https://doi.org/10.1074/JBC.M305472200
Itoh F, Itoh S, Goumans MJ et al (2004) Synergy and antagonism between Notch and BMP receptor signaling pathways in endothelial cells. EMBO J 23:541–551. https://doi.org/10.1038/SJ.EMBOJ.7600065
Blahna MT, Hata A (2012) Smad-mediatedregulation of microRNA biosynthesis. FEBS Lett 586:1906. https://doi.org/10.1016/J.FEBSLET.2012.01.041
Davis BN, Hilyard AC, Lagna G, Hata A (2008) SMAD proteins control DROSHA-mediated microRNA maturation. Nature 454:56. https://doi.org/10.1038/NATURE07086
Nohe A, Keating E, Knaus P, Petersen NO (2004) Signal transduction of bone morphogenetic protein receptors. Cell Signal 16:291–299. https://doi.org/10.1016/J.CELLSIG.2003.08.011
Zhang YE (2009) Non-Smad pathways 128 Non-Smad pathways in TGF-β signaling. Cell Res 19:128–139. https://doi.org/10.1038/cr.2008.328
Brazil DP, Church RH, Surae S et al (2015) BMP signalling: agony and antagony in the family. Trends Cell Biol 25:249–264
Walsh DW, Godson C, Brazil DP, Martin F (2010) Extracellular BMP-antagonist regulation in development and disease: tied up in knots. Trends Cell Biol 20:244–256
Groppe J, Greenwald J, Wiater E et al (2002) Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 420:636–642. https://doi.org/10.1038/NATURE01245
Lichtner B, Knaus P, Lehrach H, Adjaye J (2013) BMP10 as a potent inducer of trophoblast differentiation in human embryonic and induced pluripotent stem cells. Biomaterials 34:9789–9802. https://doi.org/10.1016/J.BIOMATERIALS.2013.08.084
Ali JL, Lagasse BJ, Minuk AJ et al (2015) Differential cellular responses induced by dorsomorphin and LDN-193189 in chemotherapy-sensitive and chemotherapy-resistant human epithelial ovarian cancer cells. Int J Cancer 136:E455–E469. https://doi.org/10.1002/IJC.29220
Hao J, Ho JN, Lewis JA et al (2010) In vivo structure—activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol 5:245–253. https://doi.org/10.1021/cb9002865
Hao J, Lee R, Chang A et al (2014) DMH1, a small molecule inhibitor of BMP type I receptors, suppresses growth and invasion of lung cancer. PLoS ONE 9:e90748. https://doi.org/10.1371/JOURNAL.PONE.0090748
Tojo M, Hamashima Y, Hanyu A et al (2005) The ALK-5 inhibitor A-83-01 inhibits Smad signaling and epithelial-to-mesenchymal transition by transforming growth factor-beta. Cancer Sci 96:791–800. https://doi.org/10.1111/J.1349-7006.2005.00103.X
Ninomiya-Tsuji J, Kajino T, Ono K et al (2003) A resorcylic acid lactone, 5Z–7-oxozeaenol, prevents inflammation by inhibiting the catalytic activity of TAK1 MAPK kinase kinase. J Biol Chem 278:18485–18490. https://doi.org/10.1074/jbc.M207453200
Wu J, Powell F, Larsen NA et al (2013) Mechanism and in vitro pharmacology of TAK1 inhibition by (5Z)-7-oxozeaenol. ACS Chem Biol 8:643–650. https://doi.org/10.1021/CB3005897
Totzke J, Gurbani D, Raphemot R et al (2017) Takinib, a selective TAK1 inhibitor, broadens the therapeutic efficacy of TNF-α inhibition for cancer and autoimmune disease. Cell Chem Biol 24:1029-1039.e7. https://doi.org/10.1016/J.CHEMBIOL.2017.07.011
Zhang H, Bradley A (1996) Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122:2977–2986. https://doi.org/10.1242/dev.122.10.2977
Lawson KA, Dunn NR, Roelen BA et al (1999) Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev 13:424–436. https://doi.org/10.1101/gad.13.4.424
Winnier G, Blessing M, Labosky PA, Hogan BL (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9:2105–2116. https://doi.org/10.1101/gad.9.17.2105
Fujiwara T, Dunn NR, Hogan BL (2001) Bone morphogenetic protein 4 in the extraembryonic mesoderm is required for allantois development and the localization and survival of primordial germ cells in the mouse. Proc Natl Acad Sci USA 98:13739–13744. https://doi.org/10.1073/pnas.241508898
Fujiwara T, Dehart DB, Sulik KK, Hogan BLM (2002) Distinct requirements for extra-embryonic and embryonic bone morphogenetic protein 4 in the formation of the node and primitive streak and coordination of left-right asymmetry in the mouse. Development 129:4685–4696. https://doi.org/10.1242/dev.129.20.4685
Soares ML, Haraguchi S, Torres-Padilla M-E et al (2005) Functional studies of signaling pathways in peri-implantation development of the mouse embryo by RNAi. BMC Dev Biol 5:28. https://doi.org/10.1186/1471-213X-5-28
Soares ML, Torres-Padilla M-E, Zernicka-Goetz M (2008) Bone morphogenetic protein 4 signaling regulates development of the anterior visceral endoderm in the mouse embryo. Dev Growth Differ 50:615–621. https://doi.org/10.1111/j.1440-169X.2008.01059.x
Cho LTY, Wamaitha SE, Tsai IJ et al (2012) Conversion from mouse embryonic to extra-embryonic endoderm stem cells reveals distinct differentiation capacities of pluripotent stem cell states. Development 139:2866–2877. https://doi.org/10.1242/dev.078519
Ying Y, Zhao GQ (2001) Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol 232:484–492. https://doi.org/10.1006/dbio.2001.0173
Di-Gregorio A, Sancho M, Stuckey DW et al (2007) BMP signalling inhibits premature neural differentiation in the mouse embryo. Development 134:3359–3369. https://doi.org/10.1242/dev.005967
Tremblay KD, Dunn NR, Robertson EJ (2001) Mouse embryos lacking Smad1 signals display defects in extra-embryonic tissues and germ cell formation. Development 128:3609–3621. https://doi.org/10.1242/dev.128.18.3609
Graham SJL, Wicher KB, Jedrusik A et al (2014) BMP signalling regulates the pre-implantation development of extra-embryonic cell lineages in the mouse embryo. Nat Commun 5:5667. https://doi.org/10.1038/ncomms6667
Home P, Saha B, Ray S et al (2012) Altered subcellular localization of transcription factor TEAD4 regulates first mammalian cell lineage commitment. Proc Natl Acad Sci USA 109:7362–7367. https://doi.org/10.1073/pnas.1201595109
Rivron NC, Frias-Aldeguer J, Vrij EJ et al (2018) Blastocyst-like structures generated solely from stem cells. Nature 557:106–111. https://doi.org/10.1038/s41586-018-0051-0
Shahbazi MN, Jedrusik A, Vuoristo S et al (2016) Self-organization of the human embryo in the absence of maternal tissues. Nat Cell Biol 18:700–708. https://doi.org/10.1038/ncb3347
Deglincerti A, Croft GF, Pietila LN et al (2016) Self-organization of the in vitro attached human embryo. Nature 533:251–254. https://doi.org/10.1038/nature17948
Blakeley P, Fogarty NME, Del Valle I et al (2015) Defining the three cell lineages of the human blastocyst by single-cell RNA-seq. Development 142:3613. https://doi.org/10.1242/dev.131235
Lee KY, Jeong J-W, Wang J et al (2007) Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol 27:5468–5478. https://doi.org/10.1128/MCB.00342-07
Monsivais D, Clementi C, Peng J et al (2017) BMP7 induces uterine receptivity and blastocyst attachment. Endocrinology 158:979–992. https://doi.org/10.1210/en.2016-1629
Monsivais D, Clementi C, Peng J et al (2016) Uterine ALK3 is essential during the window of implantation. Proc Natl Acad Sci USA 113:E387-395. https://doi.org/10.1073/pnas.1523758113
Nagashima T, Li Q, Clementi C et al (2013) BMPR2 is required for postimplantation uterine function and pregnancy maintenance. J Clin Invest 123:2539–2550. https://doi.org/10.1172/JCI65710
Stoikos CJ, Harrison CA, Salamonsen LA, Dimitriadis E (2008) A distinct cohort of the TGFbeta superfamily members expressed in human endometrium regulate decidualization. Hum Reprod 23:1447–1456. https://doi.org/10.1093/humrep/den110
Zhang Y, Zhu H, Chang H-M, Leung PCK (2020) ALK3-SMAD1/5 signaling mediates the BMP2-induced decrease in PGE2 production in human endometrial stromal cells and decidual stromal cells. Front Cell Dev Biol 8:573028. https://doi.org/10.3389/fcell.2020.573028
Pape ME, Kim KH (1988) Effect of tumor necrosis factor on acetyl-coenzyme A carboxylase gene expression and preadipocyte differentiation. Mol Endocrinol 2:395–403. https://doi.org/10.1210/mend-2-5-395
Harrison SE, Sozen B, Christodoulou N et al (2017) Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science 356:eaal1810. https://doi.org/10.1126/science.aal1810
Sozen B, Demir N, Zernicka-Goetz M (2021) BMP signalling is required for extra-embryonic ectoderm development during pre-to-post-implantation transition of the mouse embryo. Dev Biol 470:84–94. https://doi.org/10.1016/j.ydbio.2020.11.005
Yi Y, Zhu H, Klausen C et al (2021) Dysregulated BMP2 in the placenta may contribute to early-onset preeclampsia by regulating human trophoblast expression of extracellular matrix and adhesion molecules. Front Cell Dev Biol 9:768669. https://doi.org/10.3389/fcell.2021.768669
Sato N, Sanjuan IM, Heke M et al (2003) Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol 260:404–413. https://doi.org/10.1016/s0012-1606(03)00256-2
Yabe S, Alexenko AP, Amita M et al (2016) Comparison of syncytiotrophoblast generated from human embryonic stem cells and from term placentas. Proc Natl Acad Sci USA 113:E2598-2607. https://doi.org/10.1073/pnas.1601630113
Au J, Requena DF, Rishik H et al (2021) Role of autocrine bone morphogenetic protein signaling in trophoblast stem cells. Biol Reprod. https://doi.org/10.1093/biolre/ioab213
Home P, Kumar RP, Ganguly A et al (2017) Genetic redundancy of GATA factors in the extraembryonic trophoblast lineage ensures the progression of preimplantation and postimplantation mammalian development. Development 144:876–888. https://doi.org/10.1242/dev.145318
Chambers I, Smith A (2004) Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 23:7150–7160. https://doi.org/10.1038/sj.onc.1207930
Smith AG, Heath JK, Donaldson DD et al (1988) Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature 336:688–690. https://doi.org/10.1038/336688a0
Ying QL, Nichols J, Chambers I, Smith A (2003) BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 115:281–292. https://doi.org/10.1016/s0092-8674(03)00847-x
Qi X, Li T-G, Hao J et al (2004) BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA 101:6027–6032. https://doi.org/10.1073/pnas.0401367101
Gomes Fernandes M, Dries R, Roost MS et al (2016) BMP-SMAD signaling regulates lineage priming, but is dispensable for self-renewal in mouse embryonic stem cells. Stem Cell Reports 6:85–94. https://doi.org/10.1016/j.stemcr.2015.11.012
Ying Q-L, Wray J, Nichols J et al (2008) The ground state of embryonic stem cell self-renewal. Nature 453:519–523. https://doi.org/10.1038/nature06968
Nichols J, Smith A (2009) Naive and primed pluripotent states. Cell Stem Cell 4:487–492. https://doi.org/10.1016/j.stem.2009.05.015
Thomson JA, Kalishman J, Golos TG et al (1996) Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod 55:254–259. https://doi.org/10.1095/biolreprod55.2.254
Thomson JA, Itskovitz-Eldor J, Shapiro SS et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147. https://doi.org/10.1126/science.282.5391.1145
Amit M, Itskovitz-Eldor J (2006) Derivation and maintenance of human embryonic stem cells. Methods Mol Biol 331:43–53. https://doi.org/10.1385/1-59745-046-4:43
Amit M, Itskovitz-Eldor J (2006) Sources, derivation, and culture of human embryonic stem cells. Semin Reprod Med 24:298–303. https://doi.org/10.1055/s-2006-954939
James D, Levine AJ, Besser D, Hemmati-Brivanlou A (2005) TGFbeta/activin/nodal signaling is necessary for the maintenance of pluripotency in human embryonic stem cells. Development 132:1273–1282. https://doi.org/10.1242/dev.01706
Vallier L, Alexander M, Pedersen RA (2005) Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci 118:4495–4509. https://doi.org/10.1242/jcs.02553
Wei CL, Miura T, Robson P et al (2005) Transcriptome profiling of human and murine ESCs identifies divergent paths required to maintain the stem cell state. Stem Cells 23:166–185. https://doi.org/10.1634/stemcells.2004-0162
Odorico JS, Kaufman DS, Thomson JA (2001) Multilineage differentiation from human embryonic stem cell lines. Stem Cells 19:193–204. https://doi.org/10.1634/stemcells.19-3-193
Itskovitz-Eldor J, Schuldiner M, Karsenti D et al (2000) Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med 6:88–95
Xu R-H, Chen X, Li DS et al (2002) BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol 20:1261–1264. https://doi.org/10.1038/nbt761
Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc Natl Acad Sci USA 102:4783–4788. https://doi.org/10.1073/pnas.0501283102
Golos TG, Pollastrini LM, Gerami-Naini B (2006) Human embryonic stem cells as a model for trophoblast differentiation. Semin Reprod Med 24:314–321. https://doi.org/10.1055/s-2006-952154
Harun R, Ruban L, Matin M et al (2006) Cytotrophoblast stem cell lines derived from human embryonic stem cells and their capacity to mimic invasive implantation events. Hum Reprod 21:1349–1358. https://doi.org/10.1093/humrep/del017
Tesar PJ, Chenoweth JG, Brook FA et al (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448:196–199. https://doi.org/10.1038/nature05972
Brons IGM, Smithers LE, Trotter MWB et al (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448:191–195. https://doi.org/10.1038/nature05950
Huang Y, Osorno R, Tsakiridis A, Wilson V (2012) In Vivo differentiation potential of epiblast stem cells revealed by chimeric embryo formation. Cell Rep 2:1571–1578. https://doi.org/10.1016/j.celrep.2012.10.022
Takahashi S, Kobayashi S, Hiratani I (2018) Epigenetic differences between naïve and primed pluripotent stem cells. Cell Mol Life Sci 75:1191–1203. https://doi.org/10.1007/s00018-017-2703-x
Hanna JH, Saha K, Jaenisch R (2010) Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell 143:508–525. https://doi.org/10.1016/j.cell.2010.10.008
Guo G, von Meyenn F, Santos F et al (2016) Naive Pluripotent Stem Cells Derived Directly from Isolated Cells of the Human Inner Cell Mass. Stem Cell Reports 6:437–446. https://doi.org/10.1016/j.stemcr.2016.02.005
Theunissen TW, Powell BE, Wang H et al (2014) Systematic identification of culture conditions for induction and maintenance of naive human pluripotency. Cell Stem Cell 15:471–487. https://doi.org/10.1016/j.stem.2014.07.002
Weinberger L, Ayyash M, Novershtern N, Hanna JH (2016) Dynamic stem cell states: naive to primed pluripotency in rodents and humans. Nat Rev Mol Cell Biol 17:155–169. https://doi.org/10.1038/nrm.2015.28
Czyz J, Wobus A (2001) Embryonic stem cell differentiation: the role of extracellular factors. Differentiation 68:167–174. https://doi.org/10.1046/j.1432-0436.2001.680404.x
Sadlon TJ, Lewis ID, D’Andrea RJ (2004) BMP4: its role in development of the hematopoietic system and potential as a hematopoietic growth factor. Stem Cells 22:457–474. https://doi.org/10.1634/stemcells.22-4-457
Sybirna A, Wong FCK, Surani MA (2019) Genetic basis for primordial germ cells specification in mouse and human: conserved and divergent roles of PRDM and SOX transcription factors. Curr Top Dev Biol 135:35–89. https://doi.org/10.1016/bs.ctdb.2019.04.004
Aberdam D (2004) Derivation of keratinocyte progenitor cells and skin formation from embryonic stem cells. Int J Dev Biol 48:203–206. https://doi.org/10.1387/ijdb.15272386
Li Z, Chen Y-G (2013) Functions of BMP signaling in embryonic stem cell fate determination. Exp Cell Res 319:113–119. https://doi.org/10.1016/j.yexcr.2012.09.016
Sui L, Bouwens L, Mfopou JK (2013) Signaling pathways during maintenance and definitive endoderm differentiation of embryonic stem cells. Int J Dev Biol 57:1–12. https://doi.org/10.1387/ijdb.120115ls
Bernardo AS, Faial T, Gardner L et al (2011) BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell 9:144–155. https://doi.org/10.1016/j.stem.2011.06.015
Russ AP, Wattler S, Colledge WH et al (2000) Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature 404:95–99. https://doi.org/10.1038/35003601
Soncin F, Khater M, To C et al (2018) Comparative analysis of mouse and human placentae across gestation reveals species-specific regulators of placental development. Development. https://doi.org/10.1242/dev.156273
Shao Y, Taniguchi K, Gurdziel K et al (2017) Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nature Mater 16:419–425. https://doi.org/10.1038/nmat4829
Zheng Y, Xue X, Shao Y et al (2019) Controlled modelling of human epiblast and amnion development using stem cells. Nature 573:421–425. https://doi.org/10.1038/s41586-019-1535-2
Shao Y, Taniguchi K, Townshend RF et al (2017) A pluripotent stem cell-based model for post-implantation human amniotic sac development. Nat Commun 8:208. https://doi.org/10.1038/s41467-017-00236-w
Dobreva MP, Pereira PNG, Deprest J, Zwijsen A (2010) On the origin of amniotic stem cells: of mice and men. Int J Dev Biol 54:761–777. https://doi.org/10.1387/ijdb.092935md
Ma H, Zhai J, Wan H et al (2019) In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science. https://doi.org/10.1126/science.aax7890
Xiang L, Yin Y, Zheng Y et al (2020) A developmental landscape of 3D-cultured human pre-gastrulation embryos. Nature 577:537–542. https://doi.org/10.1038/s41586-019-1875-y
Roost MS, van Iperen L, Ariyurek Y et al (2015) KeyGenes, a tool to probe tissue differentiation using a human fetal transcriptional atlas. Stem Cell Rep 4:1112–1124. https://doi.org/10.1016/j.stemcr.2015.05.002
Plusa B, Frankenberg S, Chalmers A et al (2005) Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J Cell Sci 118:505–515. https://doi.org/10.1242/jcs.01666
Nishioka N, Inoue K, Adachi K et al (2009) The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 16:398–410. https://doi.org/10.1016/j.devcel.2009.02.003
Beddington RS, Robertson EJ (1989) An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development 105:733–737. https://doi.org/10.1242/dev.105.4.733
Nagy A, Gócza E, Diaz EM et al (1990) Embryonic stem cells alone are able to support fetal development in the mouse. Development 110:815–821. https://doi.org/10.1242/dev.110.3.815
Posfai E, Petropoulos S, de Barros FRO et al (2017) Position- and Hippo signaling-dependent plasticity during lineage segregation in the early mouse embryo. Elife 6:e22906. https://doi.org/10.7554/eLife.22906
Gasperowicz M, Natale DRC (2011) Establishing three blastocyst lineages—then what? Biol Reprod 84:621–630. https://doi.org/10.1095/biolreprod.110.085209
Tanaka S, Kunath T, Hadjantonakis AK et al (1998) Promotion of trophoblast stem cell proliferation by FGF4. Science 282:2072–2075. https://doi.org/10.1126/science.282.5396.2072
Erlebacher A, Price KA, Glimcher LH (2004) Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev Biol 275:158–169. https://doi.org/10.1016/j.ydbio.2004.07.032
Kubaczka C, Senner C, Araúzo-Bravo MJ et al (2014) Derivation and maintenance of murine trophoblast stem cells under defined conditions. Stem Cell Rep 2:232–242. https://doi.org/10.1016/j.stemcr.2013.12.013
Kubaczka C, Senner CE, Cierlitza M et al (2015) Direct induction of trophoblast stem cells from murine fibroblasts. Cell Stem Cell 17:557–568. https://doi.org/10.1016/j.stem.2015.08.005
Benchetrit H, Herman S, van Wietmarschen N et al (2015) Extensive nuclear reprogramming underlies lineage conversion into functional trophoblast stem-like cells. Cell Stem Cell 17:543–556. https://doi.org/10.1016/j.stem.2015.08.006
Niwa H, Miyazaki J, Smith AG (2000) Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet 24:372–376. https://doi.org/10.1038/74199
Niwa H, Toyooka Y, Shimosato D et al (2005) Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123:917–929. https://doi.org/10.1016/j.cell.2005.08.040
Peng S, Hua J, Cao X, Wang H (2011) Gelatin induces trophectoderm differentiation of mouse embryonic stem cells. Cell Biol Int 35:587–591. https://doi.org/10.1042/CBI20100452
Schenke-Layland K, Angelis E, Rhodes KE et al (2007) Collagen IV induces trophoectoderm differentiation of mouse embryonic stem cells. Stem Cells 25:1529–1538. https://doi.org/10.1634/stemcells.2006-0729
Hayashi Y, Furue MK, Tanaka S et al (2010) BMP4 induction of trophoblast from mouse embryonic stem cells in defined culture conditions on laminin. In Vitro Cell Dev Biol-Animal 46:416–430. https://doi.org/10.1007/s11626-009-9266-6
Macfarlan TS, Gifford WD, Driscoll S et al (2012) Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487:57–63. https://doi.org/10.1038/nature11244
Morgani SM, Canham MA, Nichols J et al (2013) Totipotent embryonic stem cells arise in ground-state culture conditions. Cell Rep 3:1945–1957. https://doi.org/10.1016/j.celrep.2013.04.034
Onishi K, Tonge PD, Nagy A, Zandstra PW (2014) Local BMP-SMAD1 signaling increases LIF receptor-dependent STAT3 responsiveness and primed-to-naive mouse pluripotent stem cell conversion frequency. Stem Cell Rep 3:156–168. https://doi.org/10.1016/j.stemcr.2014.04.019
Kime C, Sakaki-Yumoto M, Goodrich L et al (2016) Autotaxin-mediated lipid signaling intersects with LIF and BMP signaling to promote the naive pluripotency transcription factor program. Proc Natl Acad Sci USA 113:12478–12483. https://doi.org/10.1073/pnas.1608564113
Yu S, Zhou C, Cao S et al (2020) BMP4 resets mouse epiblast stem cells to naive pluripotency through ZBTB7A/B-mediated chromatin remodelling. Nat Cell Biol 22:651–662. https://doi.org/10.1038/s41556-020-0516-x
Kime C, Kiyonari H, Ohtsuka S et al (2019) Induced 2C expression and implantation-competent blastocyst-like cysts from primed pluripotent stem cells. Stem Cell Reports 13:485–498. https://doi.org/10.1016/j.stemcr.2019.07.011
Tomoda K, Hu H, Sahara Y et al (2021) Reprogramming epiblast stem cells into pre-implantation blastocyst cell-like cells. Stem Cell Rep 16:1197–1209. https://doi.org/10.1016/j.stemcr.2021.03.016
Guo J, Wang P, Sozen B et al (2021) Machine learning-assisted high-content analysis of pluripotent stem cell-derived embryos in vitro. Stem Cell Reports 16:1331–1346. https://doi.org/10.1016/j.stemcr.2021.03.018
Yang J, Ryan DJ, Wang W et al (2017) Establishment of mouse expanded potential stem cells. Nature 550:393–397. https://doi.org/10.1038/nature24052
Yang Y, Liu B, Xu J et al (2017) Derivation of pluripotent stem cells with in vivo embryonic and extraembryonic potency. Cell 169:243-257.e25. https://doi.org/10.1016/j.cell.2017.02.005
Posfai E, Schell JP, Janiszewski A et al (2020) Defining totipotency using criteria of increasing stringency. Cell Biol 33:991
Takahashi Y, Dominici M, Swift J et al (2006) Trophoblast stem cells rescue placental defect in SOCS3-deficient mice. J Biol Chem 281:11444–11445. https://doi.org/10.1074/jbc.C600015200
De Paepe C, Cauffman G, Verloes A et al (2013) Human trophectoderm cells are not yet committed. Hum Reprod 28:740–749. https://doi.org/10.1093/humrep/des432
Gerri C, Menchero S, Mahadevaiah SK et al (2020) Human embryogenesis: a comparative perspective. Annu Rev Cell Dev Biol 36:411–440. https://doi.org/10.1146/annurev-cellbio-022020-024900
Kunath T, Yamanaka Y, Detmar J et al (2014) Developmental differences in the expression of FGF receptors between human and mouse embryos. Placenta 35:1079–1088. https://doi.org/10.1016/j.placenta.2014.09.008
Okae H, Toh H, Sato T et al (2018) Derivation of human trophoblast stem cells. Cell Stem Cell 22:50-63.e6. https://doi.org/10.1016/j.stem.2017.11.004
Castel G, Meistermann D, Bretin B et al (2020) Induction of human trophoblast stem cells from somatic cells and pluripotent stem cells. Cell Rep 33:108419. https://doi.org/10.1016/j.celrep.2020.108419
Liu X, Ouyang JF, Rossello FJ et al (2020) Reprogramming roadmap reveals route to human induced trophoblast stem cells. Nature 586:101–107. https://doi.org/10.1038/s41586-020-2734-6
Roberts RM, Ezashi T, Sheridan MA, Yang Y (2018) Specification of trophoblast from embryonic stem cells exposed to BMP4. Biol Reprod 99:212–224. https://doi.org/10.1093/biolre/ioy070
Horii M, Touma O, Bui T, Parast MM (2020) Modeling human trophoblast, the placental epithelium at the maternal fetal interface. Reproduction 160:R1–R11. https://doi.org/10.1530/REP-19-0428
Das P, Ezashi T, Schulz LC et al (2007) Effects of fgf2 and oxygen in the bmp4-driven differentiation of trophoblast from human embryonic stem cells. Stem Cell Res 1:61–74. https://doi.org/10.1016/j.scr.2007.09.004
Li Y, Moretto-Zita M, Soncin F et al (2013) BMP4-directed trophoblast differentiation of human embryonic stem cells is mediated through a ΔNp63+ cytotrophoblast stem cell state. Development 140:3965–3976. https://doi.org/10.1242/dev.092155
Lee Y, Kim K-R, McKeon F et al (2007) A unifying concept of trophoblastic differentiation and malignancy defined by biomarker expression. Hum Pathol 38:1003–1013. https://doi.org/10.1016/j.humpath.2006.12.012
Horii M, Li Y, Wakeland AK et al (2016) Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc Natl Acad Sci USA 113:E3882-3891. https://doi.org/10.1073/pnas.1604747113
Ezashi T, Telugu BPVL, Roberts RM (2012) Model systems for studying trophoblast differentiation from human pluripotent stem cells. Cell Tissue Res 349:809–824. https://doi.org/10.1007/s00441-012-1371-2
Vallier L, Touboul T, Chng Z et al (2009) Early cell fate decisions of human embryonic stem cells and mouse epiblast stem cells are controlled by the same signalling pathways. PLoS ONE 4:e6082. https://doi.org/10.1371/journal.pone.0006082
Yu P, Pan G, Yu J, Thomson JA (2011) FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell 8:326–334. https://doi.org/10.1016/j.stem.2011.01.001
Amita M, Adachi K, Alexenko AP et al (2013) Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc Natl Acad Sci USA 110:E1212-1221. https://doi.org/10.1073/pnas.1303094110
Sudheer S, Bhushan R, Fauler B et al (2012) FGF inhibition directs BMP4-mediated differentiation of human embryonic stem cells to syncytiotrophoblast. Stem Cells Dev 21:2987–3000. https://doi.org/10.1089/scd.2012.0099
Wu Z, Zhang W, Chen G et al (2008) Combinatorial signals of activin/nodal and bone morphogenic protein regulate the early lineage segregation of human embryonic stem cells. J Biol Chem 283:24991–25002. https://doi.org/10.1074/jbc.M803893200
Erb TM, Schneider C, Mucko SE et al (2011) Paracrine and epigenetic control of trophectoderm differentiation from human embryonic stem cells: the role of bone morphogenic protein 4 and histone deacetylases. Stem Cells Dev 20:1601–1614. https://doi.org/10.1089/scd.2010.0281
Yang Y, Adachi K, Sheridan MA et al (2015) Heightened potency of human pluripotent stem cell lines created by transient BMP4 exposure. Proc Natl Acad Sci USA 112:E2337-2346. https://doi.org/10.1073/pnas.1504778112
Koel M, Võsa U, Krjutškov K et al (2017) Optimizing bone morphogenic protein 4-mediated human embryonic stem cell differentiation into trophoblast-like cells using fibroblast growth factor 2 and transforming growth factor-β/activin/nodal signalling inhibition. Reprod Biomed Online 35:253–263. https://doi.org/10.1016/j.rbmo.2017.06.003
Kurek D, Neagu A, Tastemel M et al (2015) Endogenous WNT signals mediate BMP-induced and spontaneous differentiation of epiblast stem cells and human embryonic stem cells. Stem Cell Rep 4:114–128. https://doi.org/10.1016/j.stemcr.2014.11.007
Horii M, Bui T, Touma O et al (2019) An improved two-step protocol for trophoblast differentiation of human pluripotent stem cells. Curr Protoc Stem Cell Biol. https://doi.org/10.1002/cpsc.96
Deglincerti A, Etoc F, Ozair MZ, Brivanlou AH (2016) Self-organization of spatial patterning in human embryonic stem cells. Curr Top Dev Biol 116:99–113. https://doi.org/10.1016/bs.ctdb.2015.11.010
Chhabra S, Liu L, Goh R et al (2019) Dissecting the dynamics of signaling events in the BMP, WNT, and NODAL cascade during self-organized fate patterning in human gastruloids. PLoS Biol 17:e3000498. https://doi.org/10.1371/journal.pbio.3000498
Nemashkalo A, Ruzo A, Heemskerk I, Warmflash A (2017) Morphogen and community effects determine cell fates in response to BMP4 signaling in human embryonic stem cells. Development 144:3042–3053. https://doi.org/10.1242/dev.153239
Strumpf D, Mao C-A, Yamanaka Y et al (2005) Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132:2093–2102. https://doi.org/10.1242/dev.01801
Gao X, Nowak-Imialek M, Chen X et al (2019) Establishment of porcine and human expanded potential stem cells. Nat Cell Biol 21:687–699. https://doi.org/10.1038/s41556-019-0333-2
Yu L, Wei Y, Sun H-X et al (2021) Derivation of intermediate pluripotent stem cells amenable to primordial germ cell specification. Cell Stem Cell 28:550-567.e12. https://doi.org/10.1016/j.stem.2020.11.003
Krendl C, Shaposhnikov D, Rishko V et al (2017) GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc Natl Acad Sci USA 114:E9579–E9588. https://doi.org/10.1073/pnas.1708341114
Lee CQE, Gardner L, Turco M et al (2016) What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Reports 6:257–272. https://doi.org/10.1016/j.stemcr.2016.01.006
Dong C, Beltcheva M, Gontarz P et al (2020) Derivation of trophoblast stem cells from naïve human pluripotent stem cells. Elife 9:e52504. https://doi.org/10.7554/eLife.52504
Cinkornpumin JK, Kwon SY, Guo Y et al (2020) Naive human embryonic stem cells can give rise to cells with a trophoblast-like transcriptome and methylome. Stem Cell Rep 15:198–213. https://doi.org/10.1016/j.stemcr.2020.06.003
Guo G, von Meyenn F, Rostovskaya M et al (2018) Correction: epigenetic resetting of human pluripotency. Development 145:dev166397. https://doi.org/10.1242/dev.166397
Guo G, Stirparo GG, Strawbridge SE et al (2021) Human naive epiblast cells possess unrestricted lineage potential. Cell Stem Cell 28:1040-1056.e6. https://doi.org/10.1016/j.stem.2021.02.025
Io S, Kabata M, Iemura Y et al (2021) Capturing human trophoblast development with naive pluripotent stem cells in vitro. Cell Stem Cell 28:1023-1039.e13. https://doi.org/10.1016/j.stem.2021.03.013
Chhabra S, Warmflash A (2021) BMP-treated human embryonic stem cells transcriptionally resemble amnion cells in the monkey embryo. Biol Open 10:bio058617. https://doi.org/10.1242/bio.058617
Ohgushi M, Eiraku M (2021) Cell-autonomous differentiation of human primed embryonic stem cells into trophoblastic syncytia through the nascent amnion-like cell state. Dev Biol 11:5877
Mischler A, Karakis V, Mahinthakumar J et al (2021) Two distinct trophectoderm lineage stem cells from human pluripotent stem cells. J Biol Chem 296:100386. https://doi.org/10.1016/j.jbc.2021.100386
Wei Y, Wang T, Ma L et al (2021) Efficient derivation of human trophoblast stem cells from primed pluripotent stem cells. Sci Adv 7:eabf4416. https://doi.org/10.1126/sciadv.abf4416
Li Z, Kurosawa O, Iwata H (2019) Establishment of human trophoblast stem cells from human induced pluripotent stem cell-derived cystic cells under micromesh culture. Stem Cell Res Ther 10:245. https://doi.org/10.1186/s13287-019-1339-1
Jang YJ, Kim M, Lee B-K, Kim J (2022) Induction of human trophoblast stem-like cells from primed pluripotent stem cells. Proc Natl Acad Sci USA 119:e2115709119. https://doi.org/10.1073/pnas.2115709119
Soncin F, Morey R, Bui T, Requena DF, Cheung VC, Kallol S, Kittle R, Jackson MG, Farah O, Dumdie J, Meads M, Pizzo D, Horii M, Fisch KM, Parast MM (2022) Derivation of functional trophoblast stem cells from primed human pluripotent stem cells. Stem Cell Rep 17(1303):1317
Hemberger M, Udayashankar R, Tesar P et al (2010) ELF5-enforced transcriptional networks define an epigenetically regulated trophoblast stem cell compartment in the human placenta. Hum Mol Genet 19:2456–2467. https://doi.org/10.1093/hmg/ddq128
Donker RB, Mouillet JF, Chu T et al (2012) The expression profile of C19MC microRNAs in primary human trophoblast cells and exosomes. Mol Hum Reprod 18:417–424. https://doi.org/10.1093/molehr/gas013
Pastor WA, Chen D, Liu W et al (2016) Naive human pluripotent cells feature a methylation landscape devoid of blastocyst or germline memory. Cell Stem Cell 18:323–329. https://doi.org/10.1016/j.stem.2016.01.019
Theunissen TW, Friedli M, He Y et al (2016) Molecular criteria for defining the naive human pluripotent state. Cell Stem Cell 19:502–515. https://doi.org/10.1016/j.stem.2016.06.011
Nelissen ECM, van Montfoort APA, Dumoulin JCM, Evers JLH (2011) Epigenetics and the placenta. Hum Reprod Update 17:397–417. https://doi.org/10.1093/humupd/dmq052
Hayashi Y, Ohnuma K, Furue MK (2019) Pluripotent stem cell heterogeneity. Adv Exp Med Biol 1123:71–94. https://doi.org/10.1007/978-3-030-11096-3_6
Messmer T, von Meyenn F, Savino A et al (2019) Transcriptional heterogeneity in naive and primed human pluripotent stem cells at single-cell resolution. Cell Rep 26:815-824.e4. https://doi.org/10.1016/j.celrep.2018.12.099
Polo JM, Anderssen E, Walsh RM et al (2012) A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151:1617–1632. https://doi.org/10.1016/j.cell.2012.11.039
Schwarz BA, Cetinbas M, Clement K et al (2018) Prospective isolation of poised iPSC intermediates reveals principles of cellular reprogramming. Cell Stem Cell 23:289-305.e5. https://doi.org/10.1016/j.stem.2018.06.013
Kagawa H, Javali A, Khoei HH et al (2022) Human blastoids model blastocyst development and implantation. Nature 601:600–605. https://doi.org/10.1038/s41586-021-04267-8
Yanagida A, Spindlow D, Nichols J et al (2021) Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell 28:1016-1022.e4. https://doi.org/10.1016/j.stem.2021.04.031
Sozen B, Jorgensen V, Weatherbee BAT et al (2021) Reconstructing aspects of human embryogenesis with pluripotent stem cells. Nat Commun 12:5550. https://doi.org/10.1038/s41467-021-25853-4
Fan Y, Min Z, Alsolami S et al (2021) Generation of human blastocyst-like structures from pluripotent stem cells. Cell Discov 7:81. https://doi.org/10.1038/s41421-021-00316-8
Liu X, Tan JP, Schröder J et al (2021) Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591:627–632. https://doi.org/10.1038/s41586-021-03372-y
Zhao C, Reyes AP, Schell JP et al (2021) Reprogrammed blastoids contain amnion-like cells but not trophectoderm. Dev Biol 28:1016
Karvas RM, McInturf S, Zhou J et al (2020) Use of a human embryonic stem cell model to discover GABRP, WFDC2, VTCN1 and ACTC1 as markers of early first trimester human trophoblast. Mol Hum Reprod 26:425–440. https://doi.org/10.1093/molehr/gaaa029
Hopf C, Viebahn C, Püschel B (2011) BMP signals and the transcriptional repressor BLIMP1 during germline segregation in the mammalian embryo. Dev Genes Evol. https://doi.org/10.1007/s00427-011-0373-5
Schmaltz-Panneau B, Jouneau L, Osteil P et al (2014) Contrasting transcriptome landscapes of rabbit pluripotent stem cells in vitro and in vivo. Anim Reprod Sci 149:67–79. https://doi.org/10.1016/j.anireprosci.2014.05.014
Tan T, Tang X, Zhang J et al (2011) Generation of trophoblast stem cells from rabbit embryonic stem cells with BMP4. PLoS ONE 6:17124. https://doi.org/10.1371/JOURNAL.PONE.0017124
Lee VH, Zhang SJ, Chang SMT et al (1995) In vitro transformation of rabbit cytotrophoblast cells into syncytiotrophoblast: stimulation of hormone secretion by progesterone and dibutyryl cyclic 3’,5’-adenosine monophosphate. Biol Reprod 52:868–877. https://doi.org/10.1095/BIOLREPROD52.4.868
La Rosa I (2015) Bone morphogenetic proteins in preimplantation embryos. Vitam Horm 99:223–248. https://doi.org/10.1016/BS.VH.2015.04.001
Lee K-B, Folger JK, Rajput SK, Smith GW (2014) Temporal regulation of mRNAs for select bone morphogenetic proteins (BMP), BMP receptors and their associated SMAD proteins during bovine early embryonic development: effects of exogenous BMP2 on embryo developmental progression. Reprod Biol Endocrinol 12:67. https://doi.org/10.1186/1477-7827-12-67
García EV, Miceli DC, Rizo G et al (2015) Effect of early addition of bone morphogenetic protein 5 (BMP5) to embryo culture medium on in vitro development and expression of developmentally important genes in bovine preimplantation embryos. Theriogenology 84:589–599. https://doi.org/10.1016/j.theriogenology.2015.04.018
Pennington KA, Ealy AD (2012) The expression and potential function of bone morphogenetic proteins 2 and 4 in bovine trophectoderm. Reprod Biol Endocrinol. https://doi.org/10.1186/1477-7827-10-12
Degrelle SA, Lê Cao K-A, Heyman Y et al (2011) A small set of extra-embryonic genes defines a new landmark for bovine embryo staging. Reproduction 141:79–89. https://doi.org/10.1530/REP-10-0174
La Rosa I (2015) Bone morphogenetic proteins in preimplantation embryos. Vitamins and hormones. Academic Press, Cambridge, pp 223–248
La Rosa I, Camargo LSA, Pereira MM et al (2011) Effects of bone morphogenic protein 4 (BMP4) and its inhibitor, Noggin, on in vitro maturation and culture of bovine preimplantation embryos. Reprod Biol Endocrinol 9:18. https://doi.org/10.1186/1477-7827-9-18
Suzuki Y, Koshi K, Imai K et al (2011) Bone morphogenetic protein 4 accelerates the establishment of bovine trophoblastic cell lines. Reproduction 142:733–743. https://doi.org/10.1530/REP-11-0275
Valdez Magaña G, Rodríguez A, Zhang H et al (2014) Paracrine effects of embryo-derived FGF4 and BMP4 during pig trophoblast elongation. Dev Biol 387:15–27. https://doi.org/10.1016/J.YDBIO.2014.01.008
Yuan Y, Park J, Tian Y et al (2019) A six-inhibitor culture medium for improving naïve-type pluripotency of porcine pluripotent stem cells. Cell Death Discov 5:104. https://doi.org/10.1038/s41420-019-0184-4
Cabrera-Sharp V, Read JE, Richardson S et al (2014) SMAD1/5 signaling in the early equine placenta regulates trophoblast differentiation and chorionic gonadotropin secretion. Endocrinology 155:3054–3064. https://doi.org/10.1210/en.2013-2116
Nakamura T, Okamoto I, Sasaki K, Yabuta Y, Iwatani C, Tsuchiya H, Seita Y, Nakamura S, Yamamoto T, Saitou M (2016) A developmental coordinate of pluripotency among mice, monkeys and humans. Nature 537:57–62
Kobayashi M, Takada T, Takahashi K et al (2008) BMP4 induces primitive endoderm but not trophectoderm in monkey embryonic stem cells. Cloning Stem Cells 10:495–502. https://doi.org/10.1089/CLO.2008.0030. https://home.liebertpub.com/clo
Acknowledgements
Not applicable.
Funding
This work was supported by the National Institutes of Health: R01HD089534 (M.M.P.), R01HD094937 and R21AI145071 (R.M.R.), and R01HD096260 (F.S.). J.R.O. was supported through a grant from the American Society for Reproductive Medicine.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing the first drafts of different sections of this review article, as well as to generation of Figures and Tables. RMR and MMP revised and edited the manuscript. All authors subsequently read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roberts, R.M., Ezashi, T., Temple, J. et al. The role of BMP4 signaling in trophoblast emergence from pluripotency. Cell. Mol. Life Sci. 79, 447 (2022). https://doi.org/10.1007/s00018-022-04478-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04478-w