Abstract
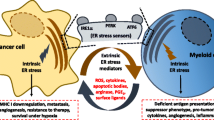
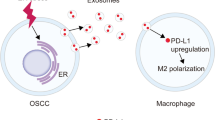
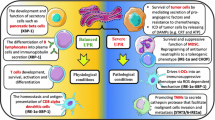
Endoplasmic reticulum (ER) stress initiates the unfolded protein response (UPR) and is decisive for tumor cell growth and tumor microenvironment (TME) maintenance. Tumor cells persistently undergo ER stress and could transmit it to the neighboring macrophages and surroundings. Tumor infiltrating macrophages can also adapt to the microenvironment variations to fulfill their highly energy-demanding and biological functions via ER stress. However, whether the different macrophage populations differentially sense ER stress and transmit ER stress to surrounding tumor cells has not yet been elucidated. Here, we aimed to investigate the role of transmissible ER stress, a novel regulator of intercellular communication in the TME. Murine bone marrow-derived macrophage (BMDM) can be polarized toward distinct functional endpoints termed classical (M1) and alternative (M2) activation, and their polarization status has been shown to be tightly correlated with their functional significance. We showed that tumor cells could receive the transmissible ER stress from two differentially polarized macrophage populations with different extent of ER stress activation. The proinflammatory M1-like macrophages respond to ER stress with less extent, however they could transmit more ER stress to tumor cells. Moreover, by analyzing the secreted components of two ER-stressed macrophage populations, we identified certain damage-associated molecular patterns (DAMPs), including S100A8 and S100A9, which are dominantly secreted by M1-like macrophages could lead to significant recipient tumor cells death in synergy with transferred ER stress.








Similar content being viewed by others
Data availability
The data generated in this study are publicly available in Gene Expression Omnibus (GEO) at GSE193669. (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE193669).
Abbreviations
- ER:
-
Endoplasmic reticulum
- TME:
-
Tumor microenvironment
- UPR:
-
Unfolded protein response
- DC:
-
Dendritic cell
- TLR:
-
Toll-like receptor
- M-CSF:
-
Macrophage colony-stimulating factor
- GM-CSF:
-
Granulocyte–macrophage colony-stimulating factor
- TAM:
-
Tumor-associated macrophage
- GM-BMDM:
-
GM-CSF cultured bone marrow-derived macrophage
- M-BMDM:
-
M-CSF cultured bone marrow-derived macrophage
- DAMP:
-
Damage-associated molecular pattern
- MAPK:
-
Mitogen-activated protein kinase
- Tm:
-
Tunicamycin
- Tg:
-
Thapsigargin
- CM:
-
Conditioned media
- Grp78:
-
Glucose-regulated protein-78
- sXbp1:
-
Spliced X-box binding protein 1
- CHOP:
-
C/EBP homologous protein
- IL:
-
Interleukin
- CXCL1:
-
C–X–C motif chemokine ligand 1
- cPARP:
-
Cleaved Poly (adp-ribose) polymerase
- p-JNK:
-
Phosphorylated c-Jun N-terminal kinase
- p-ERK:
-
Phosphorylated extracellular signal-regulated kinase
- NT:
-
No treatment
- DEG:
-
Differentially expressed gene
- GO:
-
The Gene Ontology
- IFN:
-
Interferon
- IPA:
-
Ingenuity pathway analysis
- EV:
-
Extracellular vesicle
- TNF:
-
Tumor necrosis factor
- ICD:
-
Immunogenic cell death
- DMEM:
-
Dulbecco's modified eagle medium
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- FC:
-
Fold change
References
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Diakos CI, Charles KA, McMillan DC, Clarke SJ (2014) Cancer-related inflammation and treatment effectiveness. Lancet Oncol 15:e493-503
Bettigole SE, Glimcher LH (2015) Endoplasmic reticulum stress in immunity. Annu Rev Immunol 33:107–138
Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M (2011) Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci USA 108:6561–6566
Xiong Z, Jiang R, Zhang P, Han X, Guo FJ (2015) Transmission of ER stress response by ATF6 promotes endochondral bone growth. J Orthop Surg Res 10:141
Doron B, Abdelhamed S, Butler JT, Hashmi SK, Horton TM, Kurre P (2019) Transmissible ER stress reconfigures the AML bone marrow compartment. Leukemia 33:918–930
Zhang H, Yue Y, Sun T, Wu X, Xiong S (2017) Transmissible endoplasmic reticulum stress from myocardiocytes to macrophages is pivotal for the pathogenesis of CVB3-induced viral myocarditis. Sci Rep 7:42162
Tirosh A, Tuncman G, Calay ES, Rathaus M, Ron I, Tirosh A, Yalcin A, Lee YG, Livne R, Ron S et al (2021) Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metab 33(319–333):e316
Rodvold JJ, Chiu KT, Hiramatsu N, Nussbacher JK, Galimberti V, Mahadevan NR, Willert K, Lin JH, Zanetti M (2017) Intercellular transmission of the unfolded protein response promotes survival and drug resistance in cancer cells. Sci Signal 10
Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L (2019) Macrophages and metabolism in the tumor microenvironment. Cell Metab 30:36–50
Dehne N, Mora J, Namgaladze D, Weigert A, Brune B (2017) Cancer cell and macrophage cross-talk in the tumor microenvironment. Curr Opin Pharmacol 35:12–19
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC (2008) High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 26:2707–2716
Qian BZ, Pollard JW (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–51
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, Xu D (2020) Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol 11:1731
Condeelis J, Pollard JW (2006) Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124:263–266
Lichtnekert J, Kawakami T, Parks WC, Duffield JS (2013) Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol 13:555–564
Ma J, Liu L, Che G, Yu N, Dai F, You Z (2010) The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 10:112
Hamilton TA, Zhao C, Pavicic PG Jr, Datta S (2014) Myeloid colony-stimulating factors as regulators of macrophage polarization. Front Immunol 5:554
Zhang L, Pavicic PG Jr, Datta S, Song Q, Xu X, Wei W, Su F, Rayman PA, Zhao C, Hamilton T (2019) Unfolded protein response differentially regulates TLR4-induced cytokine expression in distinct macrophage populations. Front Immunol 10:1390
Lee GY, Kenny PA, Lee EH, Bissell MJ (2007) Three-dimensional culture models of normal and malignant breast epithelial cells. Nat Methods 4:359–365
Zoetemelk M, Rausch M, Colin DJ, Dormond O, Nowak-Sliwinska P (2019) Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci Rep 9:7103
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360
Shahriyari L (2019) Effect of normalization methods on the performance of supervised learning algorithms applied to HTSeq-FPKM-UQ data sets: 7SK RNA expression as a predictor of survival in patients with colon adenocarcinoma. Brief Bioinform 20:985–994
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Brief Bioinform 15:550
Lomax J (2005) Get ready to GO! A biologist’s guide to the Gene Ontology. Brief Bioinform 6:298–304
Orlando N, Babini G, Chiusolo P, Valentini CG, De Stefano V, Teofili L (2020) Pre-exposure to defibrotide prevents endothelial cell activation by lipopolysaccharide: an ingenuity pathway analysis. Front Immunol 11:585519
Cox J, Neuhauser N, Michalski A, Scheltema RA, Olsen JV, Mann M (2011) Andromeda: a peptide search engine integrated into the MaxQuant environment. J Proteome Res 10:1794–1805
Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, Bassez A, Decaluwe H, Pircher A, Van den Eynde K et al (2018) Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 24:1277–1289
Ma L, Hernandez MO, Zhao Y, Mehta M, Tran B, Kelly M, Rae Z, Hernandez JM, Davis JL, Martin SP et al (2019) Tumor cell biodiversity drives microenvironmental reprogramming in liver cancer. Cancer Cell 36(418–430):e416
Jiang M, Li X, Zhang J, Lu Y, Shi Y, Zhu C, Liu Y, Qin B, Luo Z, Du Y et al (2021) Dual inhibition of endoplasmic reticulum stress and oxidation stress manipulates the polarization of macrophages under hypoxia to sensitize immunotherapy. ACS Nano 15:14522–14534
Wu J, Chen S, Liu H, Zhang Z, Ni Z, Chen J, Yang Z, Nie Y, Fan D (2018) Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J Exp Clin Cancer Res 37:272
Song S, Tan J, Miao Y, Li M, Zhang Q (2017) Crosstalk of autophagy and apoptosis: Involvement of the dual role of autophagy under ER stress. J Cell Physiol 232:2977–2984
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H (2014) p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett 344:174–179
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10:57–63
Tyanova S, Temu T, Cox J (2016) The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc 11:2301–2319
Andersohn A, Garcia MI, Fan Y, Thompson MC, Akimzhanov AM, Abdullahi A, Jeschke MG, Boehning D (2019) Aggregated and hyperstable damage-associated molecular patterns are released during ER stress to modulate immune function. Front Cell Dev Biol 7:198
Garg AD, Martin S, Golab J, Agostinis P (2014) Danger signalling during cancer cell death: origins, plasticity and regulation. Cell Death Differ 21:26–38
Averill MM, Kerkhoff C, Bornfeldt KE (2012) S100A8 and S100A9 in cardiovascular biology and disease. Arterioscler Thromb Vasc Biol 32:223–229
Chen X, Cubillos-Ruiz JR (2021) Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat Rev Cancer 21:71–88
Jiang Z, Zhang G, Huang L, Yuan Y, Wu C, Li Y (2020) Transmissible endoplasmic reticulum stress: a novel perspective on tumor immunity. Front Cell Dev Biol 8:846
Mahadevan NR, Anufreichik V, Rodvold JJ, Chiu KT, Sepulveda H, Zanetti M (2012) Cell-extrinsic effects of tumor ER stress imprint myeloid dendritic cells and impair CD8(+) T cell priming. PLoS ONE 7:e51845
Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, Di W (2014) A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 7:19
Barros MH, Segges P, Vera-Lozada G, Hassan R, Niedobitek G (2015) Macrophage polarization reflects T cell composition of tumor microenvironment in pediatric classical Hodgkin lymphoma and has impact on survival. PLoS ONE 10:e0124531
Boutilier AJ, Elsawa SF (2021) Macrophage polarization states in the tumor microenvironment. Int J Mol Sci 22
Wei Z, Zhang X, Yong T, Bie N, Zhan G, Li X, Liang Q, Li J, Yu J, Huang G et al (2021) Boosting anti-PD-1 therapy with metformin-loaded macrophage-derived microparticles. Nat Commun 12:440
Silini AR, Magatti M, Cargnoni A, Parolini O (2017) Is immune modulation the mechanism underlying the beneficial effects of amniotic cells and their derivatives in regenerative medicine? Cell Transplant 26:531–539
Hu G, Su Y, Kang BH, Fan Z, Dong T, Brown DR, Cheah J, Wittrup KD, Chen J (2021) High-throughput phenotypic screen and transcriptional analysis identify new compounds and targets for macrophage reprogramming. Nat Commun 12:773
Szomolay B, Eubank TD, Roberts RD, Marsh CB, Friedman A (2012) Modeling the inhibition of breast cancer growth by GM-CSF. J Theor Biol 303:141–151
Pei BX, Sun BS, Zhang ZF, Wang AL, Ren P (2014) Interstitial tumor-associated macrophages combined with tumor-derived colony-stimulating factor-1 and interleukin-6, a novel prognostic biomarker in non-small cell lung cancer. J Thorac Cardiovasc Surg 148(1208–1216):e1202
Chen YC, Lai YS, Hsuuw YD, Chang KT (2021) Withholding of M-CSF supplement reprograms macrophages to M2-like via endogenous CSF-1 activation. Int J Mol Sci 22(7):3532. https://doi.org/10.3390/ijms22073532
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I et al (2014) Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 25:846–859
Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ (2017) Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 9:347–360
Fuchs Y, Steller H (2015) Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol 16:329–344
Javeed N, Sagar G, Dutta SK, Smyrk TC, Lau JS, Bhattacharya S, Truty M, Petersen GM, Kaufman RJ, Chari ST et al (2015) Pancreatic cancer-derived exosomes cause paraneoplastic beta-cell dysfunction. Clin Cancer Res 21:1722–1733
Wu Z, Wang L, Li J, Wang L, Wu Z, Sun X (2018) Extracellular vesicle-mediated communication within host-parasite interactions. Front Immunol 9:3066
Wei C, Yang X, Liu N, Geng J, Tai Y, Sun Z, Mei G, Zhou P, Peng Y, Wang C et al (2019) Tumor microenvironment regulation by the endoplasmic reticulum stress transmission mediator golgi protein 73 in mice. Hepatology 70:851–870
Abu N, Rus Bakarurraini NAA, Nasir SN (2021) Extracellular vesicles and DAMPs in cancer: a mini-review. Front Immunol 12:740548
Ma L, Sun P, Zhang JC, Zhang Q, Yao SL (2017) Proinflammatory effects of S100A8/A9 via TLR4 and RAGE signaling pathways in BV-2 microglial cells. Int J Mol Med 40:31–38
Acknowledgements
This work was supported by the Major Program of National Natural Science Foundation of China (81991525) and Key R&D Program of Shandong Province (2020CXGC010503).
Funding
This work was supported by the Major Program of National Natural Science Foundation of China (81991525) and Key R&D Program of Shandong Province (2020CXGC010503).
Author information
Authors and Affiliations
Contributions
All authors participated in designing the study as well as interpreting the whole data. CZ and JY supervised the study; WW, YZ, XZ, ZW, XX and YY performed and evaluated individual experiments; YX and QZ performed bioinformatical analyses; WW, XL, QS, CZ and JY wrote the manuscript with the contributions from all the authors.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared no conflicts of interests.
Ethical approval
The study was approved by the Committee on the Ethics of Animal Experiments of the School of Life Sciences of Lanzhou University (EAF-2021026).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, W., Zhang, Y., Song, Q. et al. Transmissible ER stress between macrophages and tumor cells configures tumor microenvironment. Cell. Mol. Life Sci. 79, 403 (2022). https://doi.org/10.1007/s00018-022-04413-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04413-z