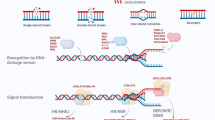
Abstract
The base excision repair (BER) pathway is essential for maintaining the stability of DNA in all organisms and defects in this process are associated with life-threatening diseases. It is involved in removing specific types of DNA lesions that are induced by both exogenous and endogenous genotoxic substances. BER is a multi-step mechanism that is often initiated by the removal of a damaged base leading to a genotoxic intermediate that is further processed before the reinsertion of the correct nucleotide and the restoration of the genome to a stable structure. Studies in human and yeast cells, as well as fruit fly and nematode worms, have played important roles in identifying the components of this conserved DNA repair pathway that maintains the integrity of the eukaryotic genome. This review will focus on the components of base excision repair, namely, the DNA glycosylases, the apurinic/apyrimidinic endonucleases, the DNA polymerase, and the ligases, as well as other protein cofactors. Functional insights into these conserved proteins will be provided from humans, Saccharomyces cerevisiae, Drosophila melanogaster, and Caenorhabditis elegans, and the implications of genetic polymorphisms and knockouts of the corresponding genes.



Similar content being viewed by others
References
Tubbs A, Nussenzweig A (2017) Endogenous DNA damage as a source of genomic instability in cancer. Cell 168(4):644–656
Matkarimov BT, Saparbaev MK (2020) DNA repair and mutagenesis in vertebrate mitochondria: evidence for asymmetric DNA strand inheritance. Adv Exp Med Biol 1241:77–100
Tremblay S, Wagner JR (2008) Dehydration, deamination and enzymatic repair of cytosine glycols from oxidized poly(dG-dC) and poly(dI-dC). Nucleic Acids Res 36(1):284–293
Boiteux S, Coste F, Castaing B (2017) Repair of 8-oxo-7,8-dihydroguanine in prokaryotic and eukaryotic cells: Properties and biological roles of the Fpg and OGG1 DNA N-glycosylases. Free Radic Biol Med 107:179–201
Slyskova J et al (2018) Base and nucleotide excision repair facilitate resolution of platinum drugs-induced transcription blockage. Nucleic Acids Res 46(18):9537–9549
Bauer NC, Corbett AH, Doetsch PW (2015) The current state of eukaryotic DNA base damage and repair. Nucleic Acids Res 43(21):10083–10101
Chatterjee N, Walker GC (2017) Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen 58(5):235–263
Moor NA, Lavrik OI (2018) Protein–protein interactions in DNA base excision repair. Biochemistry (Mosc) 83(4):411–422
Beard WA et al (2019) Eukaryotic base excision repair: new approaches shine light on mechanism. Annu Rev Biochem 88:137–162
Ayyildiz D et al (2020) Architecture of the human ape1 interactome defines novel cancers signatures. Sci Rep 10(1):28
Boiteux S, Jinks-Robertson S (2013) DNA repair mechanisms and the bypass of DNA damage in Saccharomyces cerevisiae. Genetics 193(4):1025–1064
Ljungquist S, Andersson A, Lindahl T (1974) A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. II. Further studies on the substrate specificity. J Biol Chem 249(5):1536–1540
Muench KF, Misra RP, Humayun MZ (1983) Sequence specificity in aflatoxin B1–DNA interactions. Proc Natl Acad Sci USA 80(1):6–10
Lindahl T (1993) Instability and decay of the primary structure of DNA. Nature 362(6422):709–715
Ruf A, de Murcia G, Schulz GE (1998) Inhibitor and NAD+ binding to poly(ADP-ribose) polymerase as derived from crystal structures and homology modeling. Biochemistry 37(11):3893–3900
Sobol RW et al (2000) The lyase activity of the DNA repair protein beta-polymerase protects from DNA-damage-induced cytotoxicity. Nature 405(6788):807–810
Mitra S et al (2002) Choreography of oxidative damage repair in mammalian genomes. Free Radic Biol Med 33(1):15–28
Caldecott KW (2020) Mammalian DNA base excision repair: Dancing in the moonlight. DNA Repair (Amst) 93:102921
Drohat AC, Coey CT (2016) Role of base excision “Repair” enzymes in erasing epigenetic marks from DNA. Chem Rev 116(20):12711–12729
Boldinova EO et al (2019) Isoforms of base excision repair enzymes produced by alternative splicing. Int J Mol Sci 20(13):3279
Demple B, Harrison L (1994) Repair of oxidative damage to DNA: enzymology and biology. Annu Rev Biochem 63:915–948
Muha V et al (2012) Uracil-containing DNA in Drosophila: stability, stage-specific accumulation, and developmental involvement. PLoS Genet 8(6):e1002738
Elsakrmy N, Zhang-Akiyama Q-M, Ramotar D (2020) The base excision repair pathway in the nematode caenorhabditis elegans. Front Cell Dev Biol 8(1447)
Lucas-Lledo JI, Maddamsetti R, Lynch M (2011) Phylogenomic analysis of the uracil-DNA glycosylase superfamily. Mol Biol Evol 28(3):1307–1317
Jacobs AL, Schar P (2012) DNA glycosylases: in DNA repair and beyond. Chromosoma 121(1):1–20
Sarno A et al (2019) Uracil-DNA glycosylase UNG1 isoform variant supports class switch recombination and repairs nuclear genomic uracil. Nucleic Acids Res 47(9):4569–4585
Pettersen HS et al (2007) Uracil-DNA glycosylases SMUG1 and UNG2 coordinate the initial steps of base excision repair by distinct mechanisms. Nucleic Acids Res 35(12):3879–3892
Elateri I et al (2003) hSMUG1 can functionally compensate for Ung1 in the yeast Saccharomyces cerevisiae. DNA Repair (Amst) 2(3):315–323
Schormann N, Ricciardi R, Chattopadhyay D (2014) Uracil-DNA glycosylases-structural and functional perspectives on an essential family of DNA repair enzymes. Protein Sci 23(12):1667–1685
Weiser BP et al (2018) N-terminal domain of human uracil DNA glycosylase (hUNG2) promotes targeting to uracil sites adjacent to ssDNA-dsDNA junctions. Nucleic Acids Res 46(14):7169–7178
Liu Z et al (2016) Hydrogen peroxide mediated mitochondrial UNG1-PRDX3 interaction and UNG1 degradation. Free Radic Biol Med 99:54–62
Papaluca A et al (2018) UNG-1 and APN-1 are the major enzymes to efficiently repair 5-hydroxymethyluracil DNA lesions in C elegans. Sci Rep 8(1):6860
Bekesi A et al (2007) A novel fruitfly protein under developmental control degrades uracil-DNA. Biochem Biophys Res Commun 355(3):643–648
Kuznetsova AA et al (2017) Pre-steady-state kinetic analysis of damage recognition by human single-strand selective monofunctional uracil-DNA glycosylase SMUG1. Mol Biosyst 13(12):2638–2649
An MJ et al (2021) Ablation of SMUG1 Reduces Cell Viability and Increases UVC-Mediated Apoptosis in Hepatocarcinoma HepG2 Cells. Genes (Basel) 12(2)
Kroustallaki P et al (2019) (2019) SMUG1 promotes telomere maintenance through telomerase RNA Processing. Cell Rep 28(7):1690–1702
Zhang Z et al (2016) Structural basis of substrate specificity in geobacter metallireducens SMUG1. ACS Chem Biol 11(6):1729–1736
Alsoe L et al (2017) Uracil accumulation and mutagenesis dominated by cytosine deamination in CpG dinucleotides in mice lacking UNG and SMUG1. Sci Rep 7(1):7199
Dingler FA et al (2014) Uracil excision by endogenous SMUG1 glycosylase promotes efficient Ig class switching and impacts on A: T substitutions during somatic mutation. Eur J Immunol 44(7):1925–1935
Kemmerich K et al (2012) Germline ablation of SMUG1 DNA glycosylase causes loss of 5-hydroxymethyluracil- and UNG-backup uracil-excision activities and increases cancer predisposition of Ung-/-Msh2-/- mice. Nucleic Acids Res 40(13):6016–6025
Abdel-Fatah TM et al (2013) Single-strand selective monofunctional uracil-DNA glycosylase (SMUG1) deficiency is linked to aggressive breast cancer and predicts response to adjuvant therapy. Breast Cancer Res Treat 142(3):515–527
Zhong J et al (2020) A Transcriptome-wide association study identifies novel candidate susceptibility genes for pancreatic cancer. J Natl Cancer Inst 112(10):1003–1012
Dodd T et al (2018) Uncovering universal rules governing the selectivity of the archetypal DNA glycosylase TDG. Proc Natl Acad Sci USA 115(23):5974–5979
Popov AV et al (2019) Reading targeted DNA damage in the active demethylation pathway: role of accessory domains of eukaryotic AP endonucleases and thymine-DNA glycosylases. J Mol Biol
Pidugu LS et al (2019) Excision of 5-carboxylcytosine by thymine DNA glycosylase. J Am Chem Soc 141(47):18851–18861
Bellacosa A, Drohat AC (2015) Role of base excision repair in maintaining the genetic and epigenetic integrity of CpG sites. DNA Repair (Amst) 32:33–42
DeNizio JE et al (2021) TET-TDG active DNA demethylation at CpG and non-CpG sites. J Mol Biol 433(8):166877
Deckard CE, Banerjee DR, Sczepanski JT (2019) Chromatin structure and the pioneering transcription factor FOXA1 regulate TDG-mediated removal of 5-formylcytosine from DNA. J Am Chem Soc 141(36):14110–14114
Cortazar D et al (2011) Embryonic lethal phenotype reveals a function of TDG in maintaining epigenetic stability. Nature 470(7334):419–423
Hardeland U et al (2003) The versatile thymine DNA-glycosylase: a comparative characterization of the human, Drosophila and fission yeast orthologs. Nucleic Acids Res 31(9):2261–2271
Porat N et al (2011) Direct detection of chicken genomic DNA for gender determination by thymine-DNA glycosylase. Br Poult Sci 52(1):58–65
Williams, S.C. and J.L. Parsons, NTH1 Is a New Target for Ubiquitylation-Dependent Regulation by TRIM26 Required for the Cellular Response to Oxidative Stress. Mol Cell Biol, 2018. 38(12).
Denver DR, Swenson SL, Lynch M (2003) An evolutionary analysis of the helix-hairpin-helix superfamily of DNA repair glycosylases. Mol Biol Evol 20(10):1603–1611
Morinaga H et al (2009) Purification and characterization of Caenorhabditis elegans NTH, a homolog of human endonuclease III: essential role of N-terminal region. DNA Repair (Amst) 8(7):844–851
Stehling O et al (2012) MMS19 assembles iron-sulfur proteins required for DNA metabolism and genomic integrity. Science 337(6091):195–199
Shinmura K et al (2019) Defective repair capacity of variant proteins of the DNA glycosylase NTHL1 for 5-hydroxyuracil, an oxidation product of cytosine. Free Radic Biol Med 131:264–273
Swartzlander DB et al (2016) Identification of SUMO modification sites in the base excision repair protein, Ntg1. DNA Repair (Amst) 48:51–62
Gellon L et al (2001) Synergism between base excision repair, mediated by the DNA glycosylases Ntg1 and Ntg2, and nucleotide excision repair in the removal of oxidatively damaged DNA bases in Saccharomyces cerevisiae. Mol Genet Genomics 265(6):1087–1096
Das, L., V.G. Quintana, and J.B. Sweasy, NTHL1 in genomic integrity, aging and cancer. DNA Repair (Amst), 2020. 93: p. 102920.
Lu J, Liu Y (2010) Deletion of Ogg1 DNA glycosylase results in telomere base damage and length alteration in yeast. EMBO J 29(2):398–409
Yasukawa T et al (2015) Drosophila Ogg1 is required to suppress 8-oxo-guanine accumulation following oxidative stress. Genes Genet Syst 90(1):11–20
Zhou X et al (2016) OGG1 is essential in oxidative stress induced DNA demethylation. Cell Signal 28(9):1163–1171
Ferino, A. and L.E. Xodo, Effect of DNA Glycosylases OGG1 and Neil1 on Oxidized G-Rich Motif in the KRAS Promoter. Int J Mol Sci, 2021. 22(3).
Ogawa A et al (2015) Enzyme kinetics of an alternative splicing isoform of mitochondrial 8-oxoguanine DNA glycosylase, ogg1-1b, and compared with the nuclear ogg1-1a. J Biochem Mol Toxicol 29(2):49–56
Ramdzan, Z.M., et al., CUT Domains Stimulate Pol beta Enzymatic Activities to Accelerate Completion of Base Excision Repair. J Mol Biol, 2021. 433(4): p. 166806.
Kaur S et al (2018) CUX1 stimulates APE1 enzymatic activity and increases the resistance of glioblastoma cells to the mono-alkylating agent temozolomide. Neuro Oncol 20(4):484–493
Pao PC et al (2020) HDAC1 modulates OGG1-initiated oxidative DNA damage repair in the aging brain and Alzheimer’s disease. Nat Commun 11(1):2484
Koger N et al (2019) Analysis of MUTYH alternative transcript expression, promoter function, and the effect of human genetic variants. Hum Mutat 40(4):472–482
Tan J et al (2020) An ordered assembly of MYH glycosylase, SIRT6 protein deacetylase, and Rad9-Rad1-Hus1 checkpoint clamp at oxidatively damaged telomeres. Aging (Albany NY) 12(18):17761–17785
Thibodeau, M.L., et al., Base excision repair deficiency signatures implicate germline and somatic MUTYH aberrations in pancreatic ductal adenocarcinoma and breast cancer oncogenesis. Cold Spring Harb Mol Case Stud, 2019. 5(2).
Isoda T et al (2014) Abnormality in Wnt signaling is causatively associated with oxidative stress-induced intestinal tumorigenesis in MUTYH-null mice. Int J Biol Sci 10(8):940–947
Felicio PS et al (2021) Whole-exome sequencing of non-BRCA1/BRCA2 mutation carrier cases at high-risk for hereditary breast/ovarian cancer. Hum Mutat 42(3):290–299
Sekelsky J (2017) DNA Repair in Drosophila: Mutagens, Models, and Missing Genes. Genetics 205(2):471–490
Ishchenko AA et al (2005) The 3’->5’ exonuclease of Apn1 provides an alternative pathway to repair 7,8-dihydro-8-oxodeoxyguanosine in Saccharomyces cerevisiae. Mol Cell Biol 25(15):6380–6390
Yakovlev DA et al (2017) Search for Modified DNA Sites with the Human Methyl-CpG-Binding Enzyme MBD4. Acta Naturae 9(1):88–98
Yu AM et al (2016) The Mbd4 DNA glycosylase protects mice from inflammation-driven colon cancer and tissue injury. Oncotarget 7(19):28624–28636
Zhang Y et al (2018) Epigenetic silencing of RNF144A expression in breast cancer cells through promoter hypermethylation and MBD4. Cancer Med 7(4):1317–1325
Repo P et al (2020) Germline loss-of-function variants in MBD4 are rare in Finnish patients with uveal melanoma. Pigment Cell Melanoma Res 33(5):756–762
Sanders MA et al (2018) MBD4 guards against methylation damage and germ line deficiency predisposes to clonal hematopoiesis and early-onset AML. Blood 132(14):1526–1534
Fleming AM, Burrows CJ (2017) Formation and processing of DNA damage substrates for the hNEIL enzymes. Free Radic Biol Med 107:35–52
Albelazi, M.S., et al., The Biochemical Role of the Human NEIL1 and NEIL3 DNA Glycosylases on Model DNA Replication Forks. Genes (Basel), 2019. 10(4).
Eckenroth, B.E., et al., Unique Structural Features of Mammalian NEIL2 DNA Glycosylase Prime Its Activity for Diverse DNA Substrates and Environments. Structure, 2021. 29(1): p. 29–42 e4.
Li N et al (2020) Cooperation of the NEIL3 and Fanconi anemia/BRCA pathways in interstrand crosslink repair. Nucleic Acids Res 48(6):3014–3028
Rodriguez AA et al (2020) An autoinhibitory role for the GRF zinc finger domain of DNA glycosylase NEIL3. J Biol Chem 295(46):15566–15575
Fu D, Samson LD (2012) Direct repair of 3, N(4)-ethenocytosine by the human ALKBH2 dioxygenase is blocked by the AAG/MPG glycosylase. DNA Repair (Amst) 11(1):46–52
Troll, C.J., et al., Interplay between base excision repair activity and toxicity of 3-methyladenine DNA glycosylases in an E coli complementation system. Mutat Res, 2014. 763: p. 64–73.
Fosmark S et al (2017) APNG as a prognostic marker in patients with glioblastoma. PLoS ONE 12(6):e0178693
Maher RL, Wallace SS, Pederson DS (2019) The lyase activity of bifunctional DNA glycosylases and the 3’-diesterase activity of APE1 contribute to the repair of oxidized bases in nucleosomes. Nucleic Acids Res 47(6):2922–2931
Kuznetsova AA, Fedorova OS, Kuznetsov NA (2018) Kinetic features of 3’-5’ exonuclease activity of human AP-endonuclease APE1. Molecules 23(9):2101
Ma X et al (2019) Downregulation of APE1 potentiates breast cancer cells to olaparib by inhibiting PARP-1 expression. Breast Cancer Res Treat 176(1):109–117
Kladova OA et al (2020) Modulation of the apurinic/apyrimidinic endonuclease activity of human APE1 and of its natural polymorphic variants by base excision repair proteins. Int J Mol Sci 21(19):7147
Lin Y et al (2018) APE2 promotes DNA damage response pathway from a single-strand break. Nucleic Acids Res 46(5):2479–2494
Mengwasser KE et al (2019) Genetic screens reveal FEN1 and APEX2 as BRCA2 synthetic lethal targets. Mol Cell 73(5):885–899
Gros L et al (2004) The major human AP endonuclease (Ape1) is involved in the nucleotide incision repair pathway. Nucleic Acids Res 32(1):73–81
Kuznetsova AA et al (2020) Effect of the substrate structure and metal ions on the hydrolysis of undamaged RNA by human AP endonuclease APE1. Acta Naturae 12(2):74–85
Chohan M et al (2015) Human apurinic/apyrimidinic endonuclease 1 (APE1) has 3’ RNA phosphatase and 3’ exoribonuclease activities. J Mol Biol 427(2):298–311
Nassour H et al (2016) Peroxiredoxin 1 interacts with and blocks the redox factor APE1 from activating interleukin-8 expression. Sci Rep 6:29389
Tell G, Fantini D, Quadrifoglio F (2010) Understanding different functions of mammalian AP endonuclease (APE1) as a promising tool for cancer treatment. Cell Mol Life Sci 67(21):3589–3608
Vascotto C et al (2015) Functional regulation of the apurinic/apyrimidinic endonuclease 1 by nucleophosmin: impact on tumor biology. Oncogene 34(26):3482
Kabzinski J et al (2019) Sirt3 regulates the level of mitochondrial DNA repair activity through deacetylation of NEIL1, NEIL2, OGG1, MUTYH, APE1 and LIG3 in colorectal cancer. Pol Przegl Chir 92(1):1–4
Aeby E et al (2016) Peroxiredoxin 1 protects telomeres from oxidative damage and preserves telomeric DNA for extension by telomerase. Cell Rep 17(12):3107–3114
Zhou J et al (2017) NEIL3 repairs telomere damage during s phase to secure chromosome segregation at mitosis. Cell Rep 20(9):2044–2056
Shatilla A et al (2005) Identification of two apurinic/apyrimidinic endonucleases from Caenorhabditis elegans by cross-species complementation. DNA Repair (Amst) 4(6):655–670
Sander M, Lowenhaupt K, Rich A (1991) Drosophila Rrp1 protein: an apurinic endonuclease with homologous recombination activities. Proc Natl Acad Sci USA 88(15):6780–6784
Graifer D, Karpova G (2020) Ribosomal protein uS3 in cell biology and human disease: Latest insights and prospects. BioEssays 42(12):e200124
Ramotar D et al (1991) Cellular role of yeast Apn1 apurinic endonuclease/3’-diesterase: repair of oxidative and alkylation DNA damage and control of spontaneous mutation. Mol Cell Biol 11(9):4537–4544
Johnson RE et al (1998) Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1, and its role in the repair of abasic sites. Genes Dev 12(19):3137–3143
Beard WA (2020) DNA polymerase beta: Closing the gap between structure and function. DNA Repair (Amst) 93:102910
Karimi-Busheri F et al (1998) Repair of DNA strand gaps and nicks containing 3’-phosphate and 5’-hydroxyl termini by purified mammalian enzymes. Nucleic Acids Res 26(19):4395–4400
Whitaker AM et al (2017) Base excision repair of oxidative DNA damage: from mechanism to disease. Front Biosci (Landmark Ed) 22:1493–1522
Zhao S et al (2019) Mutation in DNA polymerase beta causes spontaneous chromosomal instability and inflammation-associated carcinogenesis in mice. Cancers (Basel) 11(8):1160
Wang M et al (2020) DNA polymerase beta modulates cancer progression via enhancing CDH13 expression by promoter demethylation. Oncogene 39(33):5507–5519
Liu Y et al (2005) DNA polymerase beta and flap endonuclease 1 enzymatic specificities sustain DNA synthesis for long patch base excision repair. J Biol Chem 280(5):3665–3674
Woodrick J et al (2017) A new sub-pathway of long-patch base excision repair involving 5’ gap formation. EMBO J 36(11):1605–1622
Asagoshi K et al (2012) Single-nucleotide base excision repair DNA polymerase activity in C elegans in the absence of DNA polymerase beta. Nucleic Acids Res 40(2):670–681
Boehm EM, Gildenberg MS, Washington MT (2016) The Many roles of PCNA in eukaryotic DNA REPLICATION. Enzymes 39:231–254
Volkova NV et al (2020) Mutational signatures are jointly shaped by DNA damage and repair. Nat Commun 11(1):2169
Bebenek K et al (2005) Biochemical properties of Saccharomyces cerevisiae DNA polymerase IV. J Biol Chem 280(20):20051–20058
Jain R, Aggarwal AK, Rechkoblit O (2018) Eukaryotic DNA polymerases. Curr Opin Struct Biol 53:77–87
Gellon L et al (2008) Intrinsic 5’-deoxyribose-5-phosphate lyase activity in Saccharomyces cerevisiae Trf4 protein with a possible role in base excision DNA repair. DNA Repair (Amst) 7(2):187–198
Daley JM, Wilson TE, Ramotar D (2010) Genetic interactions between HNT3/Aprataxin and RAD27/FEN1 suggest parallel pathways for 5’ end processing during base excision repair. DNA Repair (Amst) 9(6):690–699
Sallmyr A et al (2020) Human DNA ligases in replication and repair. DNA Repair (Amst) 93:102908
Williams JS et al (2021) High-fidelity DNA ligation enforces accurate Okazaki fragment maturation during DNA replication. Nat Commun 12(1):482
Bauer RJ et al (2017) Comparative analysis of the end-joining activity of several DNA ligases. PLoS ONE 12(12):190062
Pergolizzi G, Wagner GK, Bowater RP (2016) Biochemical and Structural characterisation of DNA ligases from bacteria and archaea. Biosci Rep 36(5):00391
Lu G et al (2016) Ligase I and ligase III mediate the DNA double-strand break ligation in alternative end-joining. Proc Natl Acad Sci U S A 113(5):1256–1260
Kurosawa A, Kuboshima H, Adachi N (2020) Complex genetic interactions between DNA polymerase beta and the NHEJ ligase. FEBS J 287(2):377–385
Caglayan M, Wilson SH (2015) Oxidant and environmental toxicant-induced effects compromise DNA ligation during base excision DNA repair. DNA Repair (Amst) 35:85–89
Ismail IH et al (2015) The RNF138 E3 ligase displaces Ku to promote DNA end resection and regulate DNA repair pathway choice. Nat Cell Biol 17(11):1446–1457
Horton JK et al (2018) XRCC1 phosphorylation affects aprataxin recruitment and DNA deadenylation activity. DNA Repair (Amst) 64:26–33
Wilson DM 3rd et al (2017) Systematic analysis of DNA crosslink repair pathways during development and aging in Caenorhabditis elegans. Nucleic Acids Res 45(16):9467–9480
Gorski MM et al (2003) The Drosophila melanogaster DNA Ligase IV gene plays a crucial role in the repair of radiation-induced DNA double-strand breaks and acts synergistically with Rad54. Genetics 165(4):1929–1941
Donahue SL et al (2001) Mitochondrial DNA ligase function in Saccharomyces cerevisiae. Nucleic Acids Res 29(7):1582–1589
Arakawa H et al (2012) Functional redundancy between DNA ligases I and III in DNA replication in vertebrate cells. Nucleic Acids Res 40(6):2599–2610
Polo LM et al (2019) Efficient single-strand break repair requires binding to both poly(ADP-Ribose) and DNA by the central BRCT domain of XRCC1. Cell Rep 26(3):573–581
Reynolds P et al (2015) Disruption of PARP1 function inhibits base excision repair of a sub-set of DNA lesions. Nucleic Acids Res 43(8):4028–4038
Lavrik OI (2020) PARPs’ impact on base excision DNA repair. DNA Repair (Amst) 93:102911
Wilk A et al (2020) Extracellular NAD(+) enhances PARP-dependent DNA repair capacity independently of CD73 activity. Sci Rep 10(1):651
Sukhanova M, Khodyreva S, Lavrik O (2010) Poly(ADP-ribose) polymerase 1 regulates activity of DNA polymerase beta in long patch base excision repair. Mutat Res 685(1–2):80–89
Hanzlikova H et al (2017) Overlapping roles for PARP1 and PARP2 in the recruitment of endogenous XRCC1 and PNKP into oxidized chromatin. Nucleic Acids Res 45(5):2546–2557
Kutuzov MM et al (2021) The contribution of PARP1, PARP2 and poly(ADP-ribosyl)ation to base excision repair in the nucleosomal context. Sci Rep 11(1):4849
Mateu-Jimenez M et al (2016) Reduced tumor burden through increased oxidative stress in lung adenocarcinoma cells of PARP-1 and PARP-2 knockout mice. Biochimie 121:278–286
Abbotts R, Wilson DM 3rd (2017) Coordination of DNA single strand break repair. Free Radic Biol Med 107:228–244
Ray Chaudhuri A (2017) Nussenzweig A (2017) The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol 18(10):610–621
Velagapudi UK et al (2021) Recent development in the discovery of PARP inhibitors as anticancer agents: a patent update (2016–2020). Expert Opin Ther Pat 31:1–15
Gelmon KA et al (2021) Clinical effectiveness of olaparib monotherapy in germline BRCA-mutated, HER2-negative metastatic breast cancer in a real-world setting: phase IIIb LUCY interim analysis. Eur J Cancer 152:68–77
Dequen F, Gagnon SN, Desnoyers S (2005) Ionizing radiations in Caenorhabditis elegans induce poly(ADP-ribosyl)ation, a conserved DNA-damage response essential for survival. DNA Repair (Amst) 4(7):814–825
Crone B et al (2015) Elemental bioimaging of cisplatin in caenorhabditis elegans by LA-ICP-MS. Metallomics 7(7):1189–1195
Neumann C et al (2020) The role of poly(ADP-ribose) polymerases in manganese exposed Caenorhabditis elegans. J Trace Elem Med Biol 57:21–27
Boamah EK et al (2012) Poly(ADP-Ribose) polymerase 1 (PARP-1) regulates ribosomal biogenesis in Drosophila nucleoli. PLoS Genet 8(1):1002442
Bordet G et al (2020) Poly(ADP-ribose) polymerase 1 in genome-wide expression control in Drosophila. Sci Rep 10(1):21151
Tao Z, Gao P, Liu HW (2009) Studies of the expression of human poly(ADP-ribose) polymerase-1 in Saccharomyces cerevisiae and identification of PARP-1 substrates by yeast proteome microarray screening. Biochemistry 48(49):11745–11754
Kirby TW et al (2015) Nuclear localization of the DNA repair scaffold XRCC1: uncovering the functional role of a bipartite NLS. Sci Rep 5:13405
Cannan WJ et al (2017) The human ligase IIIalpha-XRCC1 protein complex performs DNA nick repair after transient unwrapping of nucleosomal DNA. J Biol Chem 292(13):5227–5238
Vasil’eva IA, Moor NA, Lavrik OI (2020) Effect of human XRCC1 protein oxidation on the functional activity of its complexes with the key enzymes of DNA base excision repair. Biochemistry (Mosc) 85(3):288–299
Xu C et al (2019) Deficiency of X-ray repair cross-complementing group 1 in primordial germ cells contributes to male infertility. FASEB J 33(6):7427–7436
Dutta D et al (2020) Effect of Arg399Gln single-nucleotide polymorphism in XRCC1 gene on survival rate of Indian squamous cell head-and-neck cancer patients. J Cancer Res Ther 16(3):551–558
Kaur J et al (2020) Association of XRCC1, XRCC2 and XRCC3 gene polymorphism with Esophageal cancer risk. Clin Exp Gastroenterol 13:73–86
Ghosh S et al (2015) Partial loss of the DNA repair scaffolding protein, Xrcc1, results in increased brain damage and reduced recovery from ischemic stroke in mice. Neurobiol Aging 36(7):2319–2330
Puerta-Garcia E et al (2020) Effect of DPYD, MTHFR, ABCB1, XRCC1, ERCC1 and GSTP1 on chemotherapy related toxicity in colorectal carcinoma. Surg Oncol 35:388–398
Soliman AHM et al (2020) Genetic polymorphisms in XRCC1, OGG1, and XRCC3 DNA repair genes and DNA damage in radiotherapy workers. Environ Sci Pollut Res Int 27(35):43786–43799
Fenech M et al (1991) Cloning and characterization of the rad4 gene of Schizosaccharomyces pombe; a gene showing short regions of sequence similarity to the human XRCC1 gene. Nucleic Acids Res 19(24):6737–6741
Marintchev A et al (2000) Domain specific interaction in the XRCC1-DNA polymerase beta complex. Nucleic Acids Res 28(10):2049–2059
Malfatti MC et al (2020) New perspectives in cancer biology from a study of canonical and non-canonical functions of base excision repair proteins with a focus on early steps. Mutagenesis 35(1):129–149
Sung H et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249
Ryu CS et al (2020) MPG and NPRL3 polymorphisms are associated with ischemic stroke susceptibility and post-stroke mortality. Diagnostics (Basel) 10(11):947
de Barrios O et al (2019) ZEB1 promotes inflammation and progression towards inflammation-driven carcinoma through repression of the DNA repair glycosylase MPG in epithelial cells. Gut 68(12):2129–2141
Broderick P et al (2006) Evaluation of NTHL1, NEIL1, NEIL2, MPG, TDG, UNG and SMUG1 genes in familial colorectal cancer predisposition. BMC Cancer 6:243
Ye F et al (2019) Association of SMUG1 SNPs in intron region and linkage disequilibrium with occurrence of cervical carcinoma and HPV infection in Chinese population. J Cancer 10(1):238–248
Jiang S et al. (2021) Associations of APE1 and OGG1 polymorphisms with onset and prognosis of bladder cancer. Panminerva Med
Czarny P et al (2015) Association between single nucleotide polymorphisms of MUTYH, hOGG1 and NEIL1 genes, and depression. J Affect Disord 184:90–96
Sliwinski T et al (2009) Polymorphisms of the DNA base excision repair gene MUTYH in head and neck cancer. Exp Oncol 31(1):57–59
Jensen KA, Shi X, Yan S (2020) Genomic alterations and abnormal expression of APE2 in multiple cancers. Sci Rep 10(1):3758
Wright G, Gassman NR (2020) Transcriptional dysregulation of base excision repair proteins in breast cancer. DNA Repair (Amst) 93:102922
Simon H et al (2020) OGG1 deficiency alters the intestinal microbiome and increases intestinal inflammation in a mouse model. PLoS ONE 15(1):e0227501
Mohamed RH et al (2016) Association of XRCC1 and OGG1 DNA repair gene polymorphisms with rheumatoid arthritis in Egyptian patients. Gene 578(1):112–116
Kwiatkowski D et al (2015) Variants of base excision repair genes MUTYH, PARP1 and XRCC1 in Alzheimer’s disease risk. Neuropsychobiology 71(3):176–186
Imai K et al (2003) Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol 4(10):1023–1028
Zheng L et al (2007) Fen1 mutations result in autoimmunity, chronic inflammation and cancers. Nat Med 13(7):812–819
Zhu Y et al (2020) LINC00467 is up-regulated by TDG-mediated acetylation in non-small cell lung cancer and promotes tumor progression. Oncogene 39(38):6071–6084
Yan JB et al (2020) Insulin and metformin control cell proliferation by regulating TDG-mediated DNA demethylation in liver and breast cancer cells. Mol Ther Oncolytics 18:282–294
Li D et al (2020) The HOTAIRM1/miR-107/TDG axis regulates papillary thyroid cancer cell proliferation and invasion. Cell Death Dis 11(4):227
Tomkinson AE, Naila T, Khattri BS (2020) Altered DNA ligase activity in human disease. Mutagenesis 35(1):51–60
Ke Y et al (2019) The role of PARPs in inflammation-and metabolic-related diseases: molecular mechanisms and beyond. Cells 8(9):1047
Pazzaglia S, Pioli C (2019) Multifaceted role of PARP-1 in DNA repair and inflammation: pathological and therapeutic implications in cancer and non-cancer diseases. Cells 9(1):41
Li Q, Ma R, Zhang M (2018) XRCC1 rs1799782 (C194T) polymorphism correlated with tumor metastasis and molecular subtypes in breast cancer. Onco Targets Ther 11:8435–8444
Wu W et al (2021) Neuronal enhancers are hotspots for DNA single-strand break repair. Nature
Yoon G, Caldecott KW (2018) Nonsyndromic cerebellar ataxias associated with disorders of DNA single-strand break repair. Handb Clin Neurol 155:105–115
Londero AP et al (2014) Expression and prognostic significance of APE1/Ref-1 and NPM1 proteins in high-grade ovarian serous cancer. Am J Clin Pathol 141(3):404–414
Meng L et al (2019) 2’,3’-dideoxycytidine, a DNA polymerase-beta inhibitor, reverses memory deficits in a mouse model of Alzheimer’s disease. J Alzheimers Dis 67(2):515–525
Wu Q et al (2020) Two polymorphic mutations in promoter region of DNA polymerase beta in relatively higher percentage of thymic hyperplasia patients. Thorac Cancer 12:588–592
Murphy DL et al (2012) The E288K colon tumor variant of DNA polymerase beta is a sequence specific mutator. Biochemistry 51(26):5269–5275
Acknowledgements
We thank the College of Health and Life Sciences, Hamad Bin Khalifa University, for providing scholarships to both N.N.H and N.E. The schematic representations were created using Biorender.com.
Funding
Qatar Foundation to the College of Health and Life Sciences, Hamad Bin Khalifa University, Education City, Doha, Qatar.
Author information
Authors and Affiliations
Contributions
NNH, wrote the entire manuscript using a draft provided by NE; NNH, prepared the Tables and Figures and completed the reference list; DR, extensively revised the complete manuscript and both DR and NNH finalized the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors gave their consent for publication.
Availability of data and material
Not applicable.
Conflict of interest
All the authors declared that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hindi, N.N., Elsakrmy, N. & Ramotar, D. The base excision repair process: comparison between higher and lower eukaryotes. Cell. Mol. Life Sci. 78, 7943–7965 (2021). https://doi.org/10.1007/s00018-021-03990-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-021-03990-9