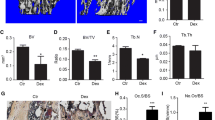
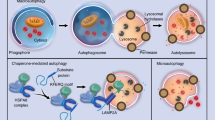
Abstract
Autophagy takes part in regulating the eukaryotic cells function and the progression of numerous diseases, but its clinical utility has not been fully developed yet. Recently, mounting evidences highlight an important correlation between autophagy and bone homeostasis, mediated by osteoclasts, osteocytes, bone marrow mesenchymal stem cells, and osteoblasts, and autophagy plays a vital role in the pathogenesis of glucocorticoid-induced osteoporosis (GIOP). The combinations of autophagy activators/inhibitors with anti-GIOP first-line drugs or some new autophagy-based manipulators, such as regulation of B cell lymphoma 2 family proteins and caspase-dependent clearance of autophagy-related gene proteins, are likely to be the promising approaches for GIOP clinical treatments. In view of the important role of autophagy in the pathogenesis of GIOP, here we review the potential mechanisms about the impacts of autophagy in GIOP and its association with GIOP therapy.




Similar content being viewed by others
References
van Staa TP, Leufkens HG, Abenhaim L, Begaud B, Zhang B, Cooper C (2000) Use of oral corticosteroids in the United Kingdom. QJM 93:105–111
Gudbjornsson B, Juliusson UI, Gudjonsson FV (2002) Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis 61:32–36
Canalis E, Mazziotti G, Giustina A, Bilezikian JP (2007) Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int 18:1319–1328
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721
Mizushima N, Komatsu M (2011) Autophagy: renovation of cells and tissues. Cell 147:728–741
Begun J, Xavier RJ (2013) Autophagy at the crossroads of metabolism and cellular defense. Curr Opin Gastroenterol 29:588–596
Mizushima N (2007) Autophagy: process and function. Genes Dev 21:2861–2873
Minina EA, Bozhkov PV, Hofius D (2014) Autophagy as initiator or executioner of cell death. Trends Plant Sci 19:692–697
Nishida K, Yamaguchi O, Otsu K (2008) Crosstalk between autophagy and apoptosis in heart disease. Circ Res 103:343–351
Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A (2005) Autophagy and aging: the importance of maintaining “clean” cells. Autophagy 1:131–140
Heraud C, Griffiths A, Pandruvada SN, Kilimann MW, Pata M, Vacher J (2014) Severe neurodegeneration with impaired autophagy mechanism triggered by ostm1 deficiency. J Biol Chem 289:13912–13925
Levine B, Mizushima N, Virgin HW (2011) Autophagy in immunity and inflammation. Nature 469:323–335
Huang YH, Al-Aidaroos AQ, Yuen HF, Zhang SD, Shen HM, Rozycka E et al (2014) A role of autophagy in PTP4A3-driven cancer progression. Autophagy 10:1787–1800
Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M et al (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13:619–624
Bo T, Yan F, Guo J, Lin X, Zhang H, Guan Q et al (2016) Characterization of a relatively malignant form of osteopetrosis caused by a novel mutation in the PLEKHM1 gene. J Bone Miner Res 31:1979–1987
Rea SL, Walsh JP, Layfield R, Ratajczak T, Xu J (2013) New insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget’s disease of bone. Endocr Rev 34:501–524
Lin NY, Chen CW, Kagwiria R, Liang R, Beyer C, Distler A et al (2016) Inactivation of autophagy ameliorates glucocorticoid-induced and ovariectomy-induced bone loss. Ann Rheum Dis 75:1203–1210
Hartmann K, Koenen M, Schauer S, Wittig-Blaich S, Ahmad M, Baschant U et al (2016) Molecular actions of glucocorticoids in cartilage and bone during health, disease, and steroid therapy. Physiol Rev 96:409–447
Florencio-Silva R, Sasso GR, Simões MJ, Simões RS, Baracat MC, Sasso-Cerri E et al (1992) Osteoporosis and autophagy: what is the relationship? Rev Assoc Med Bras 63:173–179
Fu Q, Shi H, Ren Y, Guo F, Ni W, Qiao J et al (2014) Bovine viral diarrhea virus infection induces autophagy in MDBK cells. J Microbiol 52:619–625
Dupont N, Lacas-Gervais S, Bertout J, Paz I, Freche B, Van Nhieu GT et al (2009) Shigella phagocytic vacuolar membrane remnants participate in the cellular response to pathogen invasion and are regulated by autophagy. Cell Host Microbe 6:137–149
Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931–937
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12:814–822
Yao W, Cheng Z, Busse C, Pham A, Nakamura MC, Lane NE (2008) Glucocorticoid excess in mice results in early activation of osteoclastogenesis and adipogenesis and prolonged suppression of osteogenesis: a longitudinal study of gene expression in bone tissue from glucocorticoid-treated mice. Arthritis Rheum 58:1674–1686
Tooze SA, Yoshimori T (2010) The origin of the autophagosomal membrane. Nat Cell Biol 12:831–835
Mari M, Tooze SA, Reggiori F (2011) The puzzling origin of the autophagosomal membrane F1000. Biol Rep 3:25
Kim KH, Lee MS (2014) Autophagy as a crosstalk mediator of metabolic organs in regulation of energy metabolism. Rev Endocr Metab Disord 15:11–20
Itakura E, Kishi C, Inoue K, Mizushima N (2008) Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell 19:5360–5372
Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A et al (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182:685–701
Kim KH, Lee MS (2014) Autophagy—a key player in cellular and body metabolism. Nat Rev Endocrinol 10:322–337
Mizushima N, Sugita H, Yoshimori T, Ohsumi Y (1998) A new protein conjugation system in human. The counterpart of the yeast Apg12p conjugation system essential for autophagy. J Biol Chem 273:33889–33892
Mizushima N, Noda T, Yoshimori T, Tanaka Y, Ishii T, George MD et al (1998) A protein conjugation system essential for autophagy. Nature 395:395–398
Kuma A, Mizushima N, Ishihara N, Ohsumi Y (2002) Formation of the approximately 350-kDa Apg12–Apg5.Apg16 multimeric complex, mediated by Apg16 oligomerization, is essential for autophagy in yeast. J Biol Chem 277:18619–18625
Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T (2008) The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell 19:2092–2100
Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N et al (2000) A ubiquitin-like system mediates protein lipidation. Nature 408:488–492
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T et al (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Tanida I, Sou YS, Ezaki J, Minematsu-Ikeguchi N, Ueno T, Kominami E (2004) HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three human Atg8 homologues and delipidates microtubule-associated protein light chain 3- and GABAA receptor-associated protein-phospholipid conjugates. J Biol Chem 279:36268–36276
Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326
Kuballa P, Nolte WM, Castoreno AB, Xavier RJ (2012) Autophagy and the immune system. Annu Rev Immunol 30:611–646
Fimia GM, Piacentini M (2010) Regulation of autophagy in mammals and its interplay with apoptosis. Cell Mol Life Sci 67:1581–1588
Weinstein RS (2011) Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med 365:62–70
Hofbauer LC, Rauner M (2009) Minireview: live and let die: molecular effects of glucocorticoids on bone cells. Mol Endocrinol 23:1525–1531
Weinstein RS (2012) Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin N Am 41:595–611
den Uyl D, Bultink IE, Lems WF (2011) Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep 13:233–240
Ton FN, Gunawardene SC, Lee H, Neer RM (2005) Effects of low-dose prednisone on bone metabolism. J Bone Miner Res 20:464–470
Ren H, Liang D, Shen G, Yao Z, Jiang X, Tang J et al (2016) Effects of combined ovariectomy with dexamethasone on rat lumbar vertebrae. Menopause 23:441–450
Ren H, Liang D, Jiang X, Tang J, Cui J, Wei Q et al (2015) Variance of spinal osteoporosis induced by dexamethasone and methylprednisolone and its associated mechanism. Steroids 102:65–75
Baron R, Rawadi G (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148:2635–2643
Ohnaka K, Taniguchi H, Kawate H, Nawata H, Takayanagi R (2004) Glucocorticoid enhances the expression of dickkopf-1 in human osteoblasts: novel mechanism of glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun 318:259–264
Ohnaka K, Tanabe M, Kawate H, Nawata H, Takayanagi R (2005) Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun 329:177–181
Ohnaka K (2006) Wnt signaling and glucocorticoid-induced osteoporosis. Clin Calcium 16:1812–1816
Kearns AE, Khosla S, Kostenuik PJ (2008) Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 29:155–192
Qiang YW, Chen Y, Stephens O, Brown N, Chen B, Epstein J et al (2008) Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: a potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood 112:196–207
Pinzone JJ, Hall BM, Thudi NK, Vonau M, Qiang YW, Rosol TJ et al (2009) The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood 113:517–525
Hayashi K, Yamaguchi T, Yano S, Kanazawa I, Yamauchi M, Yamamoto M et al (2009) BMP/Wnt antagonists are upregulated by dexamethasone in osteoblasts and reversed by alendronate and PTH: potential therapeutic targets for glucocorticoid-induced osteoporosis. Biochem Biophys Res Commun 379:261–266
Wang FS, Ko JY, Yeh DW, Ke HC, Wu HL (2008) Modulation of Dickkopf-1 attenuates glucocorticoid induction of osteoblast apoptosis, adipocytic differentiation, and bone mass loss. Endocrinology 149:1793–1801
La Corte R, Trotta F, Adami S (2010) Glucocorticoid receptors and bone. Curr Pharm Des 16:3586–3592
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ (2011) Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE 6:e25900
O’Brien CA, Nakashima T, Takayanagi H (2013) Osteocyte control of osteoclastogenesis. Bone 54:258–263
Kondo T, Kitazawa R, Yamaguchi A, Kitazawa S (2008) Dexamethasone promotes osteoclastogenesis by inhibiting osteoprotegerin through multiple levels. J Cell Biochem 103:335–345
Humphrey EL, Williams JH, Davie MW, Marshall MJ (2006) Effects of dissociated glucocorticoids on OPG and RANKL in osteoblastic cells. Bone 38:652–661
Rizzoli R, Biver E (2015) Glucocorticoid-induced osteoporosis: who to treat with what agent? Nat Rev Rheumatol 11:98–109
Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC (1998) Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 102:274–282
Vestergaard P, Rejnmark L, Mosekilde L (2008) Fracture risk associated with different types of oral corticosteroids and effect of termination of corticosteroids on the risk of fractures. Calcif Tissue Int 82:249–257
Compston JE (2007) Emerging consensus on prevention and treatment of glucocorticoid-induced osteoporosis. Curr Rheumatol Rep 9:78–84
Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W et al (2010) American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 62:1515–1526
Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S et al (2010) 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ 182:1864–1873
Pereira RM, Carvalho JF, Paula AP, Zerbini C, Domiciano DS, Gonçalves H et al (2012) Guidelines for the prevention and treatment of glucocorticoid-induced osteoporosis. Rev Bras Reumatol 52:580–593
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgström F, Joint IOF-ECTS GIO Guidelines Working Group et al (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23:2257–2276
Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R et al (2013) Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas 75:392–396
(2012) Actualisation 2012 des recommandations françaises du traitement médicamenteux de I’ostéoporose poménopausique (online). http://www.grio.org/documents/journee-scientifique-27-413-1390825162.pdf
National Osteoporosis Foundation (2014) Clinician’s guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington, DC
Neuerburg C, Stumpf U, Schmidmaier R, Kammerlander C, Pfeilschifter J, Mutschler W et al (2015) New DVO guideline for osteoporosis management 2014 and its importance for trauma surgeons. Unfallchirurg 118:905–912
Jia J, Yao W, Guan M, Dai W, Shahnazari M, Kar R et al (2011) Glucocorticoid dose determines osteocyte cell fate. FASEB J 25:3366–3376
Stoch SA, Wagner JA (2008) Cathepsin K inhibitors: a novel target for osteoporosis therapy. Clin Pharmacol Ther 83:172–176
Eisman JA, Bone HG, Hosking DJ, McClung MR, Reid IR, Rizzoli R et al (2011) Odanacatib in the treatment of postmenopausal women with low bone mineral density: three-year continued therapy and resolution of effect. J Bone Miner Res 26:242–251
Wang FS, Ko JY, Weng LH, Yeh DW, Ke HJ, Wu SL (2009) Inhibition of glycogen synthase kinase-3beta attenuates glucocorticoid-induced bone loss. Life Sci 85:685–692
Saag KG, Shane E, Boonen S, Marín F, Donley DW, Taylor KA et al (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357:2028–2039
Homewood CA, Warhurst DC, Peters W, Baggaley VC (1972) Lysosomes, pH and the anti-malarial action of chloroquine. Nature 235:50–52
Thome R, Lopes SC, Costa FT, Verinaud L (2013) Chloroquine: modes of action of an undervalued drug. Immunol Lett 153:50–57
White NJ (1996) The treatment of malaria. N Engl J Med 335:800–806
Singer NG, McCune WJ (1998) Update on immunosuppressive therapy. Curr Opin Rheumatol 10:169–173
Xiu Y, Xu H, Zhao C, Li J, Morita Y, Yao Z et al (2014) Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J Clin Invest 124:297–310
Lakshminarayanan S, Walsh S, Mohanraj M, Rothfield N (2001) Factors associated with low bone mineral density in female patients with systemic lupus erythematosus. J Rheumatol 28:102–108
Mok CC, Mak A, Ma KM (2005) Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 14:106–112
Pan F, Liu XG, Guo YF, Chen Y, Dong SS, Qiu C et al (2010) The regulation-of-autophagy pathway may influence Chinese stature variation: evidence from elder adults. J Hum Genet 55:441–447
Zhang L, Guo YF, Liu YZ, Liu YJ, Xiong DH, Liu XG et al (2010) Pathway-based genome-wide association analysis identified the importance of regulation-of-autophagy pathway for ultradistal radius BMD. J Bone Miner Res 25:1572–1580
Feng X, Teitelbaum SL (2013) Osteoclasts: new insights. Bone Res 1:11–26
Florencio-Silva R, Sasso GR, Sasso-Cerri E, Simões MJ, Cerri PS (2015) Biology of bone tissue: structure, function, and factors that influence bone cells. Biomed Res Int 2015:421746
Wang K, Niu J, Kim H, Kolattukudy PE (2011) Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress, and autophagy. J Mol Cell Biol 3:360–368
Sambandam Y, Townsend MT, Pierce JJ, Lipman CM, Haque A, Bateman TA et al (2014) Microgravity control of autophagy modulates osteoclastogenesis. Bone 61:125–131
Zhao Y, Chen G, Zhang W, Xu N, Zhu JY, Jia J et al (2012) Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1alpha/BNIP3 signaling pathway. J Cell Physiol 227:639–648
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS et al (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859
Nomura M, Yoshimura Y, Kikuiri T, Hasegawa T, Taniguchi Y, Deyama Y et al (2011) Platinum nanoparticles suppress osteoclastogenesis through scavenging of reactive oxygen species produced in RAW264.7 cells. J Pharmacol Sci 117:243–252
Kim MS, Yang YM, Son A, Tian YS, Lee SI, Kang SW et al (2010) RANKL-mediated reactive oxygen species pathway that induces long lasting Ca2+ oscillations essential for osteoclastogenesis. J Biol Chem 285:6913–6921
Shi J, Wang L, Zhang H, Jie Q, Li X, Shi Q et al (2015) Glucocorticoids: dose-related effects on osteoclast formation and function via reactive oxygen species and autophagy. Bone 79:222–232
Deosaran E, Larsen KB, Hua R, Sargent G, Wang Y, Kim S et al (2013) NBR1 acts as an autophagy receptor for peroxisomes. J Cell Sci 126:939–952
Bonewald L (2006) Osteocytes as multifunctional cells. J Musculoskelet Neuronal Interact 6:331–333
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241
Quarles LD (2008) Endocrine functions of bone in mineral metabolism regulation. J Clin Invest 118:3820–3828
Belanger LF (1969) Osteocytic osteolysis. Calcif Tissue Res 4:1–12
Bonewald LF (2007) Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci 1116:281–290
Feng JQ, Ye L, Schiavi S (2009) Do osteocytes contribute to phosphate homeostasis? Curr Opin Nephrol Hypertens 18:285–291
Fukumoto S (2009) The role of bone in phosphate metabolism. Mol Cell Endocrinol 310:63–70
Fukumoto S, Martin TJ (2009) Bone as an endocrine organ. Trends Endocrinol Metab 20:230–236
O’Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA et al (2004) Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology 145:1835–1841
Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T (1999) Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 104:1363–1374
Lane NE, Yao W, Balooch M, Nalla RK, Balooch G, Habelitz S et al (2006) Glucocorticoid-treated mice have localized changes in trabecular bone material properties and osteocyte lacunar size that are not observed in placebo-treated or estrogen-deficient mice. J Bone Miner Res 21:466–476
Conradie MM, de Wet H, Kotze DD, Burrin JM, Hough FS, Hulley PA (2007) Vanadate prevents glucocorticoid-induced apoptosis of osteoblasts in vitro and osteocytes in vivo. J Endocrinol 195:229–240
Yao W, Dai W, Jiang JX, Lane NE (2013) Glucocorticoids and osteocyte autophagy. Bone 54:279–284
Xia X, Kar R, Gluhak-Heinrich J, Yao W, Lane NE, Bonewald LF et al (2010) Glucocorticoid-induced autophagy in osteocytes. J Bone Miner Res 25:2479–2488
Gurusamy N, Das DK (2009) Is autophagy a double-edged sword for the heart? Acta Physiol Hung 96:267–276
Tsujimoto Y, Shimizu S (2005) Another way to die: autophagic programmed cell death. Cell Death Differ 12(Suppl 2):1528–1534
Doty SB (1981) Morphological evidence of gap junctions between bone cells. Calcif Tissue Int 33:509–512
Bejarano E, Girao H, Yuste A, Patel B, Marques C, Spray DC et al (2012) Autophagy modulates dynamics of connexins at the plasma membrane in a ubiquitin-dependent manner. Mol Biol Cell 23:2156–2169
Loiselle AE, Jiang JX, Donahue HJ (2013) Gap junction and hemichannel functions in osteocytes. Bone 54:205–212
Bivi N, Condon KW, Allen MR, Farlow N, Passeri G, Brun LR et al (2012) Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res 27:374–389
Watkins M, Grimston SK, Norris JY, Guillotin B, Shaw A, Beniash E et al (2011) Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Mol Biol Cell 22:1240–1251
Zhang Y, Paul EM, Sathyendra V, Davison A, Sharkey N, Bronson S et al (2011) Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE 6:e23516
Ishihara Y, Kamioka H, Honjo T, Ueda H, Takano-Yamamoto T, Yamashiro T (2008) Hormonal, pH, and calcium regulation of connexin 43-mediated dye transfer in osteocytes in chick calvaria. J Bone Miner Res 23:350–360
Schmelzle T, Hall MN (2000) TOR, a central controller of cell growth. Cell 103:253–262
Gao J, Cheng TS, Qin A, Pavlos NJ, Wang T, Song K et al (2016) Glucocorticoid impairs cell–cell communication by autophagy-mediated degradation of connexin 43 in osteocytes. Oncotarget 7:26966–26978
Piemontese M, Onal M, Xiong J, Wang Y, Almeida M, Thostenson JD et al (2015) Suppression of autophagy in osteocytes does not modify the adverse effects of glucocorticoids on cortical bone. Bone 75:18–26
Arvidson K, Abdallah BM, Applegate LA, Baldini N, Cenni E, Gomez-Barrena E et al (2011) Bone regeneration and stem cells. J Cell Mol Med 15:718–746
Bosch P, Musgrave DS, Lee JY, Cummins J, Shuler T, Ghivizzani TC et al (2000) Osteoprogenitor cells within skeletal muscle. J Orthop Res 18:933–944
Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV (1966) Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 16:381–390
Jones E, Churchman SM, English A, Buch MH, Horner EA, Burgoyne CH et al (2010) Mesenchymal stem cells in rheumatoid synovium: enumeration and functional assessment in relation to synovial inflammation level. Ann Rheum Dis 69:450–457
Lin CS, Xin ZC, Deng CH, Ning H, Lin G, Lue TF (2010) Defining adipose tissue-derived stem cells in tissue and in culture. Histol Histopathol 25:807–815
Hocking LJ, Whitehouse C, Helfrich MH (2012) Autophagy: a new player in skeletal maintenance? J Bone Miner Res 27:1439–1447
Wang L, Fan J, Lin YS, Guo YS, Gao B, Shi QY et al (2015) Glucocorticoids induce autophagy in rat bone marrow mesenchymal stem cells. Mol Med Rep 11:2711–2716
Song IH, Caplan AI, Dennis JE (2009) Dexamethasone inhibition of confluence-induced apoptosis in human mesenchymal stem cells. J Orthop Res 27:216–221
Xiao Y, Peperzak V, van Rijn L, Borst J, de Bruijn JD (2010) Dexamethasone treatment during the expansion phase maintains stemness of bone marrow mesenchymal stem cells. J Tissue Eng Regen Med 4:374–386
Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A (2009) Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ 16:966–975
Zhang Q, Yang YJ, Wang H, Dong QT, Wang TJ, Qian HY et al (2012) Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cells Dev 21:1321–1332
Wang L, Zhang HY, Gao B, Shi J, Huang Q, Han YH et al (2017) Tetramethylpyrazine protects against glucocorticoid-induced apoptosis by promoting autophagy in mesenchymal stem cells and improves bone mass in glucocorticoid-induced osteoporosis rats. Stem Cells Dev 26:419–430
Li X, Warmington KS, Niu QT, Asuncion FJ, Barrero M, Grisanti M et al (2010) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 25:2647–2656
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A et al (2014) Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med 370:412–420
Yao W, Dai W, Jiang L, Lay EY, Zhong Z, Ritchie RO et al (2016) Sclerostin-antibody treatment of glucocorticoid-induced osteoporosis maintained bone mass and strength. Osteoporos Int 27:283–294
Levy JMM, Towers CG, Thorburn A (2017) Targeting autophagy in cancer. Nat Rev Cancer 17:528–542
Sciarretta S, Maejima Y, Zablocki D, Sadoshima J (2017) The role of autophagy in the heart. Annu Rev Physiol. https://doi.org/10.1146/annurev-physiol-021317-121427
Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M et al (2014) Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 112:24–49
Qian W, Liu J, Jin J, Ni W, Xu W (2007) Arsenic trioxide induces not only apoptosis but also autophagic cell death in leukemia cell lines via up-regulation of Beclin-1. Leuk Res 31:329–339
Levine B, Yuan J (2005) Autophagy in cell death: an innocent convict? J Clin Invest 115:2679–2688
Oberstein A, Jeffrey PD, Shi Y (2007) Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem 282:13123–13132
Wei Y, Pattingre S, Sinha S, Bassik M, Levine B (2008) JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell 30:678–688
Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I et al (2010) Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis 1:e18
Yousefi S, Perozzo R, Schmid I, Ziemiecki A, Schaffner T, Scapozza L et al (2006) Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat Cell Biol 8:1124–1132
Betin VM, Lane JD (2009) Atg4D at the interface between autophagy and apoptosis. Autophagy 5:1057–1059
Acknowledgements
This study was jointly funded by the Natural Science Foundation of China (nos. 81503591, 81774338, 81674000), Science and Technology Projects of Guangdong Province (nos. 2014A020221021, 2016A020226006), Natural Science Foundation of Guangdong Province (no. 2014A030310082), Excellent Doctor Project of the First School of Clinic Medicine of Guangzhou University of Chinese Medicine (nos. YB201602, YB201501).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, G., Ren, H., Shang, Q. et al. Autophagy as a target for glucocorticoid-induced osteoporosis therapy. Cell. Mol. Life Sci. 75, 2683–2693 (2018). https://doi.org/10.1007/s00018-018-2776-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-018-2776-1