Abstract
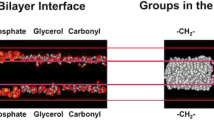
Magnetic resonance results, principally from 2H-nuclear magnetic resonance, indicate that the mean lipid-chain ordering at the surface of transmembrane proteins is comparable to that in fluid lipid bilayers. Principally, it is the requirement for matching the hydrophobic lengths of lipid and protein that modulates the degree of chain ordering at the lipid-protein interface. The distribution of chain order parameters is, nonetheless, broader in the presence of integral proteins than in fluid lipid bilayers. The chain configurations of the phospholipids that are resolved in crystals of integral membrane proteins display considerable conformational heterogeneity. Chain C–C dihedral angles are, however, not restricted to the energetically allowable trans and gauche rotamers. This indicates that the chains of a given lipid do not have a unique configuration in protein crystals.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marsh, D. Lipid interactions with transmembrane proteins. CMLS, Cell. Mol. Life Sci. 60, 1575–1580 (2003). https://doi.org/10.1007/s00018-003-3171-z
Issue Date:
DOI: https://doi.org/10.1007/s00018-003-3171-z