Abstract
Background
The pathogen responsible for tuberculosis is called Mycobacterium tuberculosis. Its interaction with macrophages has a significant impact on the onset and progression of the disease.
Methods
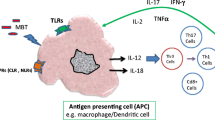
The respiratory pathway allows Mycobacterium tuberculosis to enter the body's lungs where it battles immune cells before being infected latently or actively. In the progress of tuberculosis, Mycobacterium tuberculosis activates the body's immune system and creates inflammatory factors, which cause tissue inflammation to infiltrate and the creation of granulomas, which seriously harms the body. Toll-like receptors of macrophage can mediate host recognition of Mycobacterium tuberculosis, initiate immune responses, and participate in macrophage autophagy. New host-directed therapeutic approaches targeting autophagy for drug-resistant Mycobacterium tuberculosis have emerged, providing new ideas for the effective treatment of tuberculosis.
Conclusions
In-depth understanding of the mechanisms by which macrophage autophagy interacts with intracellular Mycobacterium tuberculosis, as well as the study of potent and specific autophagy-regulating molecules, will lead to much-needed advances in drug discovery and vaccine design, which will improve the prevention and treatment of human tuberculosis.

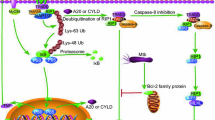
Note: Toll-like receptors, which identify molecules with conserved structures derived from MTB, are a significant class of protein molecules involved in non-specific immunity. TLR1, TLR2, TLR4, and LTR6 are surface receptors on macrophages. TLR2 can work in concert with TLR1 and TLR6 to recognize molecules of various MTB PMAPs combinatorially. Also, TLR4 is capable of identifying multiple MTB ligands. TLR3, TLR7, TLR8, and TLR9 located in the endosomal/lysosomal compartment and are involved in the recognition of nucleic acid components of MTB

Note: TLR2 forms a heterodimer with TLR1/TLR6 upon identification of the macrophage surface Toll-like receptor by MTB ligands, hence initiating the MyD88-dependent pathway. TLR7/8 and TLR9 also cause the MyD88-dependent pathway to become active. When TLR4 recognizes the appropriate ligands, it activates both the MyD88-dependent and MyD88-independent pathways (TRIF pathway). Only the MyD88 independent pathway is triggered by TLR3. The TIR structural domain of TLRs changes shape when the extracellular region identifies the matching PAMPs, and the C-terminus keeps attracting MyD88 or other junctional proteins. After IRAK is recruited by MyD88 through KD, downstream signaling via TRAF6, TAK1, TAB1, and 2 is started, activating NF-κB or AP-1 and causing the production of inflammatory cytokines. MyD88 non-dependent pathway activation via TRIF and TRAF3 leads to IKKε/TBK1 recruitment, IRF3 phosphorylation and IFN-β expression
Similar content being viewed by others
Data availability
The data that support the findings of this review are openly available in public resources.
References
Global tuberculosis report 2023. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO.
Ufimtseva EG, Eremeeva NI, Umpeleva TV, Vakhrusheva DV, Skornyakov SN. Mycobacterium tuberculosis load in host cells and the antibacterial activity of alveolar macrophages are linked and differentially regulated in various lung lesions of patients with pulmonary tuberculosis. IJMS. 2021;22(7):3452. https://doi.org/10.3390/ijms22073452.
Mubarak RA. Comparison of pro- and anti-inflammatory responses in paired human primary airway epithelial cells and alveolar macrophages. Respir Res. 2018;19:126.
Delgado MA, Elmaoued RA, Davis AS, Kyei G, Deretic V. Toll-like receptors control autophagy. EMBO J. 2008;27(7):1110–21. https://doi.org/10.1038/emboj.2008.31.
Xu Y, Fattah EA, Liu XD, Jagannath C, Eissa NT. Harnessing of TLR-mediated autophagy to combat mycobacteria in macrophages. Tuberculosis (Edinb). 2013;93(Suppl):S33-37. https://doi.org/10.1016/S1472-9792(13)70008-8.
von Both U, Berk M, Agapow PM, et al. Mycobacterium tuberculosis exploits a molecular off switch of the immune system for intracellular survival. Sci Rep. 2018;8(1):661. https://doi.org/10.1038/s41598-017-18528-y.
Franzenburg S, Fraune S, Kunzel S, Baines JF, Domazet-Loso T, Bosch TCG. MyD88-deficient Hydra reveal an ancient function of TLR signaling in sensing bacterial colonizers. Proc Natl Acad Sci. 2012;109(47):19374–9. https://doi.org/10.1073/pnas.1213110109.
Liu H, Yang M, Tang X, et al. Molecular insights of a novel fish Toll-like receptor 9 homologue in Nibea albiflora to reveal its function as PRRs. Fish Shellfish Immunol. 2021;118:321–32. https://doi.org/10.1016/j.fsi.2021.09.021.
Shepardson KM, Schwarz B, Larson K, et al. Induction of antiviral immune response through recognition of the repeating subunit pattern of viral capsids is Toll-like receptor 2 dependent. MBio. 2017;8(6): e01356-17. https://doi.org/10.1128/mBio.01356-17.
Gosu V, Son S, Shin D, Song KD. Insights into the dynamic nature of the dsRNA-bound TLR3 complex. Sci Rep. 2019;9(1):3652. https://doi.org/10.1038/s41598-019-39984-8.
Yu L, Phillips RL, Zhang D, Teghanemt A, Weiss JP, Gioannini TL. NMR studies of hexaacylated endotoxin bound to wild-type and F126A mutant MD-2 and MD-2·TLR4 ectodomain complexes. J Biol Chem. 2012;287(20):16346–55. https://doi.org/10.1074/jbc.M112.343467.
Voogdt CGP, Wagenaar JA, van Putten JPM. Duplicated TLR5 of zebrafish functions as a heterodimeric receptor. Proc Natl Acad Sci USA. 2018;115(14):E3221–9. https://doi.org/10.1073/pnas.1719245115.
Zhang S, Hu Z, Tanji H, et al. Small-molecule inhibition of TLR8 through stabilization of its resting state. Nat Chem Biol. 2018;14(1):58–64. https://doi.org/10.1038/nchembio.2518.
Fuchs K, Cardona Gloria Y, Wolz O, et al. The fungal ligand chitin directly binds TLR 2 and triggers inflammation dependent on oligomer size. EMBO Rep. 2018;19(12):e46065. https://doi.org/10.15252/embr.201846065.
Bermudez M, Grabowski M, Murgueitio MS, et al. Biological characterization, mechanistic investigation and structure-activity relationships of chemically stable TLR2 antagonists. ChemMedChem. 2020;15(14):1364–71. https://doi.org/10.1002/cmdc.202000060.
Huang WC, Liou CJ, Shen SC, et al. Urolithin A inactivation of TLR3/TRIF signaling to block the NF-κB/STAT1 axis reduces inflammation and enhances antioxidant defense in poly(I:C)-induced RAW264.7 cells. Int J Mol Sci. 2022;23(9):4697. https://doi.org/10.3390/ijms23094697.
Anderson JA, Loes AN, Waddell GL, Harms MJ. 2009 Tracing the evolution of novel features of human Toll-like receptor 4. Protein Sci. 2019;28:1350–8. https://doi.org/10.1002/pro.3644.
Kwon JO, Jin WJ, Kim B, Ha H, Kim HH, Lee ZH. Haptoglobin acts as a TLR4 ligand to suppress osteoclastogenesis via the TLR4–IFN-β axis. J Immunol. 2019;202(12):3359–69. https://doi.org/10.4049/jimmunol.1800661.
Nicholas SA, Coughlan K, Yasinska I, et al. Dysfunctional mitochondria contain endogenous high-affinity human Toll-like receptor 4 (TLR4) ligands and induce TLR4-mediated inflammatory reactions. Int J Biochem Cell Biol. 2011;43(4):674–81. https://doi.org/10.1016/j.biocel.2011.01.012.
Lei X, Palomero J, de Rink I, et al. Flagellin/TLR5 stimulate myeloid progenitors to enter lung tissue and to locally differentiate into macrophages. Front Immunol. 2021;12: 621665. https://doi.org/10.3389/fimmu.2021.621665.
Lendeckel U, Venz S, Wolke C. Macrophages: shapes and functions. ChemTexts. 2022;8(2):12. https://doi.org/10.1007/s40828-022-00163-4.
Wong AO, Marthi M, Haag A, Owusu IA, Wobus CE, Swanson JA. Macrophage inflammatory state influences susceptibility to lysosomal damage. J Leukoc Biol. 2022;111(3):629–39. https://doi.org/10.1002/JLB.3A0520-325RR.
Lim YJ, Yi MH, Choi JA, et al. Roles of endoplasmic reticulum stress-mediated apoptosis in M1-polarized macrophages during mycobacterial infections. Sci Rep. 2016;6:37211. https://doi.org/10.1038/srep37211.
Fortingo N, Melnyk S, Sutton SH, Watsky MA, Bollag WB. Innate immune system activation, inflammation and corneal wound healing. Int J Mol Sci. 2022;23(23):14933. https://doi.org/10.3390/ijms232314933.
Dunston CR, Griffiths HR. The effect of ageing on macrophage Toll-like receptor-mediated responses in the fight against pathogens. Clin Exp Immunol. 2010;161(3):407–16. https://doi.org/10.1111/j.1365-2249.2010.04213.x.
Liu CH, Liu H, Ge B. Innate immunity in tuberculosis: host defense vs pathogen evasion. Cell Mol Immunol. 2017;14(12):963–75. https://doi.org/10.1038/cmi.2017.88.
Fitzgerald KA, Kagan JC. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–66. https://doi.org/10.1016/j.cell.2020.02.041.
Irizarry-Caro RA, McDaniel MM, Overcast GR, Jain VG, Troutman TD, Pasare C. TLR signaling adapter BCAP regulates inflammatory to reparatory macrophage transition by promoting histone lactylation. Proc Natl Acad Sci USA. 2020;117(48):30628–38. https://doi.org/10.1073/pnas.2009778117.
Veltkamp M, van Moorsel CHM, Rijkers GT, Ruven HJT, Grutters JC. Genetic variation in the Toll-like receptor gene cluster (TLR10-TLR1-TLR6) influences disease course in sarcoidosis. Tissue Antigens. 2012;79(1):25–32. https://doi.org/10.1111/j.1399-0039,2011.01808.x.
Shukla S, Richardson ET, Drage MG, Boom WH, Harding CV. Mycobacterium tuberculosis lipoprotein and lipoglycan binding to Toll-like receptor 2 correlates with agonist activity and functional outcomes. Infect Immun. 2018;86(10): e00450-18. https://doi.org/10.1128/IAI.00450-18.
Hu W, Yang S, Shimada Y, et al. Infection and RNA-seq analysis of a zebrafish tlr2 mutant shows a broad function of this toll-like receptor in transcriptional and metabolic control and defense to Mycobacterium marinum infection. BMC Genomics. 2019;20(1):878. https://doi.org/10.1186/s12864-019-6265-1.
Kielbik M, Szulc-Kielbik I, Klink M. IRAK1 and IRAK4 signaling proteins are dispensable in the response of human neutrophils to Mycobacterium tuberculosis infection. FEMS Microbiol Lett. 2019;366(18):fnz226. https://doi.org/10.1093/femsle/fnz226.
Ernst O, Vayttaden SJ, Fraser IDC. Measurement of NF-κB activation in TLR-activated macrophages. In: De Nardo D, De Nardo CM, editors. Innate immune activation. Vol 1714. Methods in molecular biology. New York: Springer; 2018, pp. 67–78. https://doi.org/10.1007/978-1-4939-7519-8_5.
Alvarez-Jiménez VD, Leyva-Paredes K, García-Martínez M, et al. Extracellular vesicles released from Mycobacterium tuberculosis-infected neutrophils promote macrophage autophagy and decrease intracellular mycobacterial survival. Front Immunol. 2018;9:272. https://doi.org/10.3389/fimmu.2018.00272.
Szulc-Kielbik I, Kielbik M, Przygodzka P, Brzostek A, Dziadek J, Klink M. Mycobacterium tuberculosis requires cholesterol oxidase to disrupt TLR2 signalling in human macrophages. Mediators Inflamm. 2019;2019:1–17. https://doi.org/10.1155/2019/2373791.
Bednarska K, Kielbik M, Sulowska Z, Dziadek J, Klink M. Cholesterol oxidase binds TLR2 and modulates functional responses of human macrophages. Mediators Inflamm. 2014;2014: 498395. https://doi.org/10.1155/2014/498395.
Klink M, Brzezinska M, Szulc I, et al. Cholesterol oxidase is indispensable in the pathogenesis of Mycobacterium tuberculosis. PLoS ONE. 2013;8(9): e73333. https://doi.org/10.1371/journal.pone.0073333.
Palucci I, Camassa S, Cascioferro A, et al. PE_PGRS33 contributes to Mycobacterium tuberculosis entry in macrophages through interaction with TLR2. PLoS ONE. 2016;11(3): e0150800. https://doi.org/10.1371/journal.pone.0150800.
Minerva M, De Maio F, Camassa S, et al. Evaluation of PE_PGRS33 as a potential surface target for humoral responses against Mycobacterium tuberculosis. Pathog Dis. 2017;75(8):1093–100. https://doi.org/10.1093/femspd/ftx100.
Basu S, Pathak SK, Banerjee A, et al. Execution of macrophage apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by Toll-like receptor 2-dependent release of tumor necrosis factor-alpha. J Biol Chem. 2007;282(2):1039–50. https://doi.org/10.1074/jbc.M604379200.
Liu Y, Li JY, Chen ST, Huang HR, Cai H. The rLrp of Mycobacterium tuberculosis inhibits proinflammatory cytokine production and downregulates APC function in mouse macrophages via a TLR2-mediated PI3K/Akt pathway activation-dependent mechanism. Cell Mol Immunol. 2016;13(6):729–46. https://doi.org/10.1038/cmi.2015.58.
Pattanaik KP, Ganguli G, Naik SK, Sonawane A. Mycobacterium tuberculosis EsxL induces TNF-α secretion through activation of TLR2 dependent MAPK and NF-κB pathways. Mol Immunol. 2021;130:133–41. https://doi.org/10.1016/j.molimm.2020.11.020.
Su H, Zhang Z, Liu Z, et al. Mycobacterium tuberculosis PPE60 antigen drives Th1/Th17 responses via Toll-like receptor 2-dependent maturation of dendritic cells. J Biol Chem. 2018;293(26):10287–302. https://doi.org/10.1074/jbc.RA118.001696.
Su H, Kong C, Zhu L, et al. PPE26 induces TLR2-dependent activation of macrophages and drives Th1-type T-cell immunity by triggering the cross-talk of multiple pathways involved in the host response. Oncotarget. 2015;6(36):38517–37. https://doi.org/10.18632/oncotarget.5956.
Yihao D, Hongyun H, Maodan T. Latency-associated protein Rv2660c of Mycobacterium tuberculosis augments expression of proinflammatory cytokines in human macrophages by interacting with TLR2. Infect Dis (Lond). 2015;47(3):168–77. https://doi.org/10.3109/00365548.2014.982167.
Li W, Deng W, Zhang N, Peng H, Xu Y. Mycobacterium tuberculosis Rv2387 facilitates mycobacterial survival by silencing TLR2/p38/JNK signaling. Pathogens. 2022;11(9):981. https://doi.org/10.3390/pathogens11090981.
Manjunath P, Ahmad J, Samal J, et al. Mycobacterium tuberculosis specific protein Rv1509 evokes efficient innate and adaptive immune response indicative of protective Th1 immune signature. Front Immunol. 2021;12: 706081. https://doi.org/10.3389/fimmu.2021.706081.
Gao X, Wu C, He W, et al. DosR antigen Rv1737c induces activation of macrophages dependent on the TLR2 pathway. Cell Immunol. 2019;344: 103947. https://doi.org/10.1016/j.cellimm.2019.103947.
Su H, Zhu S, Zhu L, et al. Recombinant lipoprotein Rv1016c derived from Mycobacterium tuberculosis is a TLR-2 ligand that induces macrophages apoptosis and inhibits MHC II antigen processing. Front Cell Infect Microbiol. 2016;6:147. https://doi.org/10.3389/fcimb.2016.00147.
Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci USA. 1998;95(2):588–93. https://doi.org/10.1073/pnas.95.2.588.
Datta SK, Redecke V, Prilliman KR, et al. A subset of Toll-like receptor ligands induces cross-presentation by bone marrow-derived dendritic cells. J Immunol. 2003;170(8):4102–10. https://doi.org/10.4049/jimmunol.170.8.4102.
Lv HL, Yu J, Pei JF, Wang HY, Guo ZL. TIR-domain-containing adapter-inducing interferon-β contributes to TLR3/TLR4 triggered apoptosis and inflammation in nucleus pulposus cells. J Biol Regul Homeost Agents. 2020;34(2):445–55. https://doi.org/10.23812/20-66-A-35.
Bai W, Liu H, Ji Q, et al. TLR3 regulates mycobacterial RNA-induced IL-10 production through the PI3K/AKT signaling pathway. Cell Signal. 2014;26(5):942–50. https://doi.org/10.1016/j.cellsig.2014.01.015.
Abel B, Thieblemont N, Quesniaux VJF, et al. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J Immunol. 2002;169(6):3155–62. https://doi.org/10.4049/jimmunol.169.6.3155.
Mazurek J, Ignatowicz L, Kallenius G, Svenson SB, Pawlowski A, Hamasur B. Divergent effects of mycobacterial cell wall glycolipids on maturation and function of human monocyte-derived dendritic cells. Kovats S, ed. PLoS ONE. 2012;7(8):e42515. https://doi.org/10.1371/journal.pone.0042515.
Jang AR, Choi JH, Shin SJ, Park JH. Mycobacterium tuberculosis ESAT6 induces IFN-β gene expression in macrophages via TLRs-mediated signaling. Cytokine. 2018;104:104–9. https://doi.org/10.1016/j.cyto.2017.10.006.
Pahari S, Negi S, Aqdas M, Arnett E, Schlesinger LS, Agrewala JN. Induction of autophagy through CLEC4E in combination with TLR4: an innovative strategy to restrict the survival of Mycobacterium tuberculosis. Autophagy. 2020;16(6):1021–43. https://doi.org/10.1080/15548627.2019.1658436.
Choi HG, Choi S, Back YW, et al. Mycobacterium tuberculosis Rv2882c protein induces activation of macrophages through TLR4 and exhibits vaccine potential. PLoS ONE. 2016;11(10): e0164458. https://doi.org/10.1371/journal.pone.0164458.
Kim K, Sohn H, Kim JS, et al. Mycobacterium tuberculosis Rv0652 stimulates production of tumour necrosis factor and monocytes chemoattractant protein-1 in macrophages through the Toll-like receptor 4 pathway. Immunology. 2012;136(2):231–40. https://doi.org/10.1111/j.1365-2567.2012.03575.x.
Shariq M, Quadir N, Sharma N, et al. Mycobacterium tuberculosis RipA dampens TLR4-mediated host protective response using a multi-pronged approach involving autophagy, apoptosis, metabolic repurposing, and immune modulation. Front Immunol. 2021;12: 636644. https://doi.org/10.3389/fimmu.2021.636644.
Nehvi IB, Quadir N, Khubaib M, et al. ArgD of Mycobacterium tuberculosis is a functional N-acetylornithine aminotransferase with moonlighting function as an effective immune modulator. Int J Med Microbiol. 2022;312(1): 151544. https://doi.org/10.1016/j.ijmm.2021.151544.
Quadir N, Shariq M, Sheikh JA, et al. Mycobacterium tuberculosis protein MoxR1 enhances virulence by inhibiting host cell death pathways and disrupting cellular bioenergetics. Virulence. 2023;14(1):2180230. https://doi.org/10.1080/21505594.2023.2180230.
Bhatt P, Sharma M, Prakash Sharma P, Rathi B, Sharma S. Mycobacterium tuberculosis dormancy regulon proteins Rv2627c and Rv2628 as Toll like receptor agonist and as potential adjuvant. Int Immunopharmacol. 2022;112: 109238. https://doi.org/10.1016/j.intimp.2022.109238.
Park HS, Back YW, Shin KW, et al. Mycobacterium tuberculosis Rv3463 induces mycobactericidal activity in macrophages by enhancing phagolysosomal fusion and exhibits therapeutic potential. Sci Rep. 2019;9(1):4246. https://doi.org/10.1038/s41598-019-38982-0.
Lee KI, Choi S, Choi HG, et al. Recombinant Rv3261 protein of Mycobacterium tuberculosis induces apoptosis through a mitochondrion-dependent pathway in macrophages and inhibits intracellular bacterial growth. Cell Immunol. 2020;354: 104145. https://doi.org/10.1016/j.cellimm.2020.104145.
Lee KI, Choi S, Choi HG, et al. Recombinant Rv1654 protein of Mycobacterium tuberculosis induces mitochondria-mediated apoptosis in macrophage. Microbiol Immunol. 2021;65(4):178–88. https://doi.org/10.1111/1348-0421.12880.
Medha, Priyanka, Bhatt P, Sharma S, Sharma M. Role of C-terminal domain of Mycobacterium tuberculosis PE6 (Rv0335c) protein in host mitochondrial stress and macrophage apoptosis. Apoptosis. 2023;28(1–2):136–65. https://doi.org/10.1007/s10495-022-01778-1.
Sharma T, Singh J, Grover S, et al. PGRS domain of Rv0297 of Mycobacterium tuberculosis functions in a calcium dependent manner. Int J Mol Sci. 2021;22(17):9390. https://doi.org/10.3390/ijms22179390.
Grover S, Sharma T, Singh Y, et al. The PGRS domain of Mycobacterium tuberculosis PE_PGRS Protein Rv0297 is involved in endoplasmic reticulum stress-mediated apoptosis through Toll-like receptor 4. MBio. 2018;9(3): e01017-18. https://doi.org/10.1128/mBio.01017-18.
Hs P, Yw B, It J, et al. Mycobacterium tuberculosis Rv2145c promotes intracellular survival by STAT3 and IL-10 receptor signaling. Front Immunol. 2021;12: 666293. https://doi.org/10.3389/fimmu.2021.666293.
Lai YF, Lin TM, Wang CH, et al. Functional polymorphisms of the TLR7 and TLR8 genes contribute to Mycobacterium tuberculosis infection. Tuberculosis (Edinb). 2016;98:125–31. https://doi.org/10.1016/j.tube.2016.03.008.
Bakhru P, Sirisaengtaksin N, Soudani E, Mukherjee S, Khan A, Jagannath C. BCG vaccine mediated reduction in the MHC-II expression of macrophages and dendritic cells is reversed by activation of Toll-like receptors 7 and 9. Cell Immunol. 2014;287(1):53–61. https://doi.org/10.1016/j.cellimm.2013.11.007.
Pawar K, Shigematsu M, Sharbati S, Kirino Y. Infection-induced 5’-half molecules of tRNA His GUG activate Toll-like receptor 7. PLoS Biol. 2020;18(12): e3000982. https://doi.org/10.1371/journal.pbio.3000982.
Liu YC, Simmons DP, Li X, Abbott DW, Boom WH, Harding CV. TLR2 signaling depletes IRAK1 and inhibits induction of type I IFN by TLR7/9. J Immunol. 2012;188(3):1019–26. https://doi.org/10.4049/jimmunol.1102181.
Nguyen H, Gazy N, Venketaraman V. A role of intracellular Toll-like receptors (3, 7, and 9) in response to Mycobacterium tuberculosis and co-infection with HIV. Int J Mol Sci. 2020;21(17):6148. https://doi.org/10.3390/ijms21176148.
Chuang TH, Ulevitch RJ. Cloning and characterization of a sub-family of human toll-like receptors: hTLR7, hTLR8 and hTLR9. Eur Cytokine Netw. 2000;11(3):372–8.
Tang J, Sun M, Shi G, et al. Toll-like receptor 8 agonist strengthens the protective efficacy of ESAT-6 immunization to Mycobacterium tuberculosis Infection. Front Immunol. 2018;8:1972. https://doi.org/10.3389/fimmu.2017.01972.
Salie M, Daya M, Lucas LA, et al. Association of toll-like receptors with susceptibility to tuberculosis suggests sex-specific effects of TLR8 polymorphisms. Infect Genet Evol. 2015;34:221–9. https://doi.org/10.1016/j.meegid.2015.07.004.
Keegan C, Krutzik S, Schenk M, et al. Mycobacterium tuberculosis transfer RNA induces IL-12p70 via synergistic activation of pattern recognition receptors within a cell network. J Immunol. 2018;200(9):3244–58. https://doi.org/10.4049/jimmunol.1701733.
Tang J, Zhan L, Qin C. Inhibition of TLR8 mediated signaling promotes BCG induced apoptosis in THP-1 cells. Microb Pathog. 2016;93:78–82. https://doi.org/10.1016/j.micpath.2015.11.028.
Wang MG, Zhang MM, Wang Y, Wu SQ, Zhang M, He JQ. Association of TLR8 and TLR9 polymorphisms with tuberculosis in a Chinese Han population: a case-control study. BMC Infect Dis. 2018;18(1):561. https://doi.org/10.1186/s12879-018-3485-y.
Jin Y, Zhuang Y, Dong X, Liu M. Development of CpG oligodeoxynucleotide TLR9 agonists in anti-cancer therapy. Expert Rev Anticancer Ther. 2021;21(8):841–51. https://doi.org/10.1080/14737140.2021.1915136.
Choi SS, Chung E, Jung YJ. Newly identified CpG ODNs, M5–30 and M6–395, stimulate mouse immune cells to secrete TNF-alpha and enhance Th1-mediated immunity. J Microbiol. 2010;48(4):512–7. https://doi.org/10.1007/s12275-010-0053-6.
Zyzak J, Mitkiewicz M, Leszczyńska E, Reniewicz P, Moynagh PN, Siednienko J. HSV-1/TLR9-mediated IFNβ and TNFα induction is mal-dependent in macrophages. J Innate Immun. 2020;12(5):387–98. https://doi.org/10.1159/000504542.
Li J, Fu L, Wang G, Subbian S, Qin C, Zhao A. Unmethylated CpG motif-containing genomic DNA fragment of Bacillus calmette-guerin promotes macrophage functions through TLR9-mediated activation of NF-κB and MAPKs signaling pathways. Innate Immun. 2020;26(3):183–203. https://doi.org/10.1177/1753425919879997.
Ohto U, Ishida H, Shibata T, Sato R, Miyake K, Shimizu T. Toll-like receptor 9 contains two DNA binding sites that function cooperatively to promote receptor dimerization and activation. Immunity. 2018;48(4):649-658.e4. https://doi.org/10.1016/j.immuni.2018.03.013.
Ruiz A, Guzmán-Beltrán S, Carreto-Binaghi LE, Gonzalez Y, Juárez E. DNA from virulent M. tuberculosis induces TNF-α production and autophagy in M1 polarized macrophages. Microb Pathog. 2019;132:166–77. https://doi.org/10.1016/j.micpath.2019.04.041.
Troy A, Esparza-Gonzalez SC, Bartek A, Creissen E, Izzo L, Izzo AA. Pulmonary mucosal immunity mediated through CpG provides adequate protection against pulmonary Mycobacterium tuberculosis infection in the mouse model. A role for type I interferon. Tuberculosis (Edinb. 2020;123: 101949. https://doi.org/10.1016/j.tube.2020.101949.
Coelho da Silva FD, Covre LP, Stringari LL, et al. Toll-like receptors blocking restores in vitro microbicidal activity in latent tuberculosis-infected subjects. Int J Tuberc Lung Dis. 2019;23(2):212–8. https://doi.org/10.5588/ijtld.18.0392.
Li W, He P, Huang Y, et al. Selective autophagy of intracellular organelles: recent research advances. Theranostics. 2021;11(1):222–56. https://doi.org/10.7150/thno.49860.
Mesquita A, Glenn J, Jenny A. Differential activation of eMI by distinct forms of cellular stress. Autophagy. 2021;17(8):1828–40. https://doi.org/10.1080/15548627.2020.1783833.
Park JM, Kim DH. A paradigm shift: AMPK negatively regulates ULK1 activity. Autophagy. 2023;1–3. https://doi.org/10.1080/15548627.2023.2223465.
Nitta A, Hori K, Tanida I, et al. Blocking LC3 lipidation and ATG12 conjugation reactions by ATG7 mutant protein containing C572S. Biochem Biophys Res Commun. 2019;508(2):521–6. https://doi.org/10.1016/j.bbrc.2018.11.158.
Iriondo MN, Alonso A. Vesicle tethering and fusion promoted by LC3/GABARAP proteins is modulated by the ATG12-ATG5-ATG16L1 complex. Autophagy. 2023;19(10):2827–9. https://doi.org/10.1080/15548627.2023.2202557.
Seto S, Tsujimura K, Horii T, Koide Y. Autophagy adaptor protein p62/SQSTM1 and autophagy-related gene Atg5 mediate autophagosome formation in response to Mycobacterium tuberculosis infection in dendritic cells. PLoS ONE. 2013;8(12): e86017. https://doi.org/10.1371/journal.pone.0086017.
Abreu S, Kriegenburg F, Gómez-Sánchez R, et al. Conserved Atg8 recognition sites mediate Atg4 association with autophagosomal membranes and Atg8 deconjugation. EMBO Rep. 2017;18(5):765–80. https://doi.org/10.15252/embr.201643146.
Esteves AR, Filipe F, Magalhães JD, Silva DF, Cardoso SM. The role of Beclin-1 acetylation on autophagic flux in Alzheimer’s disease. Mol Neurobiol. 2019;56(8):5654–70. https://doi.org/10.1007/s12035-019-1483-8.
Yang Z, Huang J, Geng J, Nair U, Klionsky DJ. Atg22 recycles amino acids to link the degradative and recycling functions of autophagy. Mol Biol Cell. 2006;17(12):5094–104. https://doi.org/10.1091/mbc.e06-06-0479.
Kimmey JM, Huynh JP, Weiss LA, et al. Unique role for ATG5 in PMN-mediated immunopathology during M. tuberculosis infection. Nature. 2015;528(7583):565–9. https://doi.org/10.1038/nature16451.
Aylan B, Bernard EM, Pellegrino E, et al. ATG7 and ATG14 restrict cytosolic and phagosomal Mycobacterium tuberculosis replication in human macrophages. Nat Microbiol. 2023;8(5):803–18. https://doi.org/10.1038/s41564-023-01335-9.
Deng W, Long Q, Zeng J, et al. Mycobacterium tuberculosis PE_PGRS41 enhances the intracellular survival of M. smegmatis within macrophages via blocking innate immunity and inhibition of host defense. Sci Rep. 2017;7:46716. https://doi.org/10.1038/srep46716.
Wu Y, Lin X, Song F, Xue D, Wang Y. Vitamin D3 promotes autophagy in THP-1 cells infected with Mycobacterium tuberculosis. Exp Ther Med. 2022;23(3):240. https://doi.org/10.3892/etm.2022.11165.
Dong W, Wang G, Feng J, et al. MiR-25 blunts autophagy and promotes the survival of Mycobacterium tuberculosis by regulating NPC1. iScience. 2022;25(5): 104279. https://doi.org/10.1016/j.isci.2022.104279.
Sharma D, Tiwari BK, Mehto S, et al. Suppression of protective responses upon activation of L-type voltage gated calcium channel in macrophages during Mycobacterium bovis BCG infection. PLoS ONE. 2016;11(10): e0163845. https://doi.org/10.1371/journal.pone.0163845.
Lee HJ, Kang SJ, Woo Y, Hahn TW, Ko HJ, Jung YJ. TLR7 stimulation with imiquimod induces selective autophagy and controls Mycobacterium tuberculosis growth in mouse macrophages. Front Microbiol. 2020;11:1684. https://doi.org/10.3389/fmicb.2020.01684.
Zhang Q, Sun J, Wang Y, et al. Antimycobacterial and anti-inflammatory mechanisms of Baicalin via induced autophagy in macrophages infected with Mycobacterium tuberculosis. Front Microbiol. 2017;8:2142. https://doi.org/10.3389/fmicb.2017.02142.
Gluschko A, Farid A, Herb M, Grumme D, Krönke M, Schramm M. Macrophages target Listeria monocytogenes by two discrete non-canonical autophagy pathways. Autophagy. 2022;18(5):1090–107. https://doi.org/10.1080/15548627.2021.1969765.
Köster S, Upadhyay S, Chandra P, et al. Mycobacterium tuberculosis is protected from NADPH oxidase and LC3-associated phagocytosis by the LCP protein CpsA. Proc Natl Acad Sci USA. 2017;114(41):E8711–20. https://doi.org/10.1073/pnas.1707792114.
Hu D, Wu J, Zhang R, et al. Autophagy-targeted vaccine of LC3-LpqH DNA and its protective immunity in a murine model of tuberculosis. Vaccine. 2014;32(20):2308–14. https://doi.org/10.1016/j.vaccine.2014.02.069.
Shin DM, Yuk JM, Lee HM, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010;12(11):1648–65. https://doi.org/10.1111/j.1462-5822.2010.01497.x.
Strong EJ, Wang J, Ng TW, Porcelli SA, Lee S. Mycobacterium tuberculosis PPE51 inhibits autophagy by suppressing Toll-like receptor 2-dependent signaling. MBio. 2022;13(3): e0297421. https://doi.org/10.1128/mbio.02974-21.
Bah A, Sanicas M, Nigou J, Guilhot C, Astarie-Dequeker C, Vergne I. The lipid virulence factors of Mycobacterium tuberculosis exert multilayered control over autophagy-related pathways in infected human macrophages. Cells. 2020;9(3):E666. https://doi.org/10.3390/cells9030666.
Padhi A, Pattnaik K, Biswas M, Jagadeb M, Behera A, Sonawane A. Mycobacterium tuberculosis LprE suppresses TLR2-dependent cathelicidin and autophagy expression to enhance bacterial survival in macrophages. J Immunol. 2019;203(10):2665–78. https://doi.org/10.4049/jimmunol.1801301.
Shi CS, Kehrl JH. MyD88 and Trif target Beclin 1 to trigger autophagy in macrophages. J Biol Chem. 2008;283(48):33175–82. https://doi.org/10.1074/jbc.M804478200.
Sharma N, Shariq M, Quadir N, et al. Mycobacterium tuberculosis protein PE6 (Rv0335c), a novel TLR4 agonist, evokes an inflammatory response and modulates the cell death pathways in macrophages to enhance intracellular survival. Front Immunol. 2021;12: 696491. https://doi.org/10.3389/fimmu.2021.696491.
Bao M, Yi Z, Fu Y. Activation of TLR7 inhibition of Mycobacterium tuberculosis survival by autophagy in RAW 264.7 macrophages. J Cell Biochem. 2017;118(12):4222–9. https://doi.org/10.1002/jcb.26072.
Bonilla DL, Bhattacharya A, Sha Y, et al. Autophagy regulates phagocytosis by modulating the expression of scavenger receptors. Immunity. 2013;39(3):537–47. https://doi.org/10.1016/j.immuni.2013.08.026.
Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150(4):803–15. https://doi.org/10.1016/j.cell.2012.06.040.
Dong H, Jing W, Runpeng Z, et al. ESAT6 inhibits autophagy flux and promotes BCG proliferation through MTOR. Biochem Biophys Res Commun. 2016;477(2):195–201. https://doi.org/10.1016/j.bbrc.2016.06.042.
Huang D, Bao L. Mycobacterium tuberculosis EspB protein suppresses interferon-γ-induced autophagy in murine macrophages. J Microbiol Immunol Infect. 2016;49(6):859–65. https://doi.org/10.1016/j.jmii.2014.11.008.
Romagnoli A, Etna MP, Giacomini E, et al. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012;8(9):1357–70. https://doi.org/10.4161/auto.20881.
Chatterjee S, Dwivedi VP, Singh Y, et al. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a Toll-like receptor-2-dependent manner. PLoS Pathog. 2011;7(11): e1002378. https://doi.org/10.1371/journal.ppat.1002378.
Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119(6):753–66. https://doi.org/10.1016/j.cell.2004.11.038.
Chen W, Liu Z, Zheng Y, et al. Selenium donor restricts the intracellular growth of Mycobacterium tuberculosis through the induction of c-Jun-mediated both canonical autophagy and LC3-associated phagocytosis of alveolar macrophages. Microb Pathog. 2021;161: 105269. https://doi.org/10.1016/j.micpath.2021.105269.
Kim TS, Jin YB, Kim YS, et al. SIRT3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy. 2019;15(8):1356–75. https://doi.org/10.1080/15548627.2019.1582743.
Wang J, Sha J, Strong E, Chopra AK, Lee S. FDA-approved amoxapine effectively promotes macrophage control of mycobacteria by inducing autophagy. Microbiol Spectr. 2022;10(5): e0250922. https://doi.org/10.1128/spectrum.02509-22.
Kim JJ, Lee HM, Shin DM, et al. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe. 2012;11(5):457–68. https://doi.org/10.1016/j.chom.2012.03.008.
Singh DK, Bhaskar A, Pahuja I, et al. Cotreatment with clofazimine and rapamycin eliminates drug-resistant tuberculosis by inducing polyfunctional central memory T-cell responses. J Infect Dis. 2023;228(9):1166–78. https://doi.org/10.1093/infdis/jiad214.
Bhatt K, Bhagavathula M, Verma S, et al. Rapamycin modulates pulmonary pathology in a murine model of Mycobacterium tuberculosis infection. Dis Model Mech. 2021;14(10): dmm049018. https://doi.org/10.1242/dmm.049018.
Hussain T, Zhao D, Shah SZA, et al. Nilotinib: a tyrosine kinase inhibitor mediates resistance to intracellular Mycobacterium via regulating autophagy. Cells. 2019;8(5):506. https://doi.org/10.3390/cells8050506.
Schiebler M, Brown K, Hegyi K, et al. Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol Med. 2015;7(2):127–39. https://doi.org/10.15252/emmm.201404137.
Chen G, Wu B, Wu M, Liu F, Qin C, Luo W. Autophagy-related genes affect drug resistance of mycobacteria by regulating autophagy. Int J Clin Exp Pathol. 2019;12(6):2001–8.
Rao Muvva J, Ahmed S, Rekha RS, et al. Immunomodulatory agents combat multidrug-resistant tuberculosis by improving antimicrobial immunity. J Infect Dis. 2021;224(2):332–44. https://doi.org/10.1093/infdis/jiab100.
Kan LLY, Liu D, Chan BCL, et al. The flavonoids of Sophora flavescens exerts anti-inflammatory activity via promoting autophagy of Bacillus Calmette-Guérin-stimulated macrophages. J Leukoc Biol. 2020;108(5):1615–29. https://doi.org/10.1002/JLB.3MA0720-682RR.
Gupta PK, Jahagirdar P, Tripathi D, Devarajan PV, Kulkarni S. Macrophage targeted polymeric curcumin nanoparticles limit intracellular survival of Mycobacterium tuberculosis through induction of autophagy and augment anti-TB activity of isoniazid in RAW 264.7 macrophages. Front Immunol. 2023;14:1233630. https://doi.org/10.3389/fimmu.2023.1233630.
Lee YJ, Kim JK, Jung CH, et al. Chemical modulation of SQSTM1/p62-mediated xenophagy that targets a broad range of pathogenic bacteria. Autophagy. 2022;18(12):2926–45. https://doi.org/10.1080/15548627.2022.2054240.
Boland R, Heemskerk MT, Forn-Cuní G, et al. Repurposing tamoxifen as potential host-directed therapeutic for tuberculosis. MBio. 2023;14(1): e0302422. https://doi.org/10.1128/mbio.03024-22.
Singh S, Yabaji SM, Ali R, Srivastava KK, Haq W. Synthesis and biological activity of Ub2 derived peptides as potential host-directed antitubercular therapy. Chem Biol Drug Des. 2019;94(1):1330–8. https://doi.org/10.1111/cbdd.13508.
Giraud-Gatineau A, Coya JM, Maure A, et al. The antibiotic bedaquiline activates host macrophage innate immune resistance to bacterial infection. Elife. 2020;9: e55692. https://doi.org/10.7554/eLife.55692.
Khan N, Pahari S, Vidyarthi A, Aqdas M, Agrewala JN. Stimulation through CD40 and TLR-4 is an effective host directed therapy against Mycobacterium tuberculosis. Front Immunol. 2016;7:386. https://doi.org/10.3389/fimmu.2016.00386.
Sánchez A, Espinosa P, García T, Mancilla R. The 19 kDa Mycobacterium tuberculosis lipoprotein (LpqH) induces macrophage apoptosis through extrinsic and intrinsic pathways: a role for the mitochondrial apoptosis-inducing factor. Clin Dev Immunol. 2012;2012: 950503. https://doi.org/10.1155/2012/950503.
Acknowledgements
We acknowledge the funding support from the National Nature Science Foundation of China (NSFC) (No. 81860291).
Author information
Authors and Affiliations
Contributions
Junmin Luo, Jihong Feng contributed to the conception of the review; Linna Wei wrote the manuscript; Liping Liu performed manuscript preparation contributed collection; Zudi Meng, Kai Qi, Xuehan Gao helped perform constructive discussions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Mauro Teixeira.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, L., Liu, L., Meng, Z. et al. Recognition of Mycobacterium tuberculosis by macrophage Toll-like receptor and its role in autophagy. Inflamm. Res. 73, 753–770 (2024). https://doi.org/10.1007/s00011-024-01864-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-024-01864-x