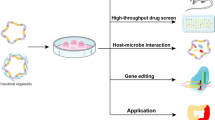
Abstract
Background
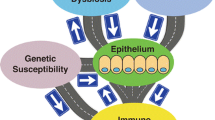
Inflammatory bowel disease (IBD) is an increasingly prevalent global health concern that has garnered substantial attention. However, the underlying mechanisms are still unclear and the current treatments have significant limitations. Intestinal organoids provide an in vitro model to explore the pathogenesis, test the therapeutic effects, and develop regenerative treatments as well as offer the potential to transform drug discovery of IBD.
Methods
To advance our understanding of the whole story of IBD spanning from the pathogenesis to the current therapeutic strategies and latest advancements, a comprehensive search of major databases including PubMed, Scopus, and Web of Science was conducted to retrieve original articles and reviews related to IBD, organoids, pathogenesis and therapy.
Results
This review deciphers the etiopathogenesis and the current therapeutic approaches in the treatment of IBD. Notably, critical aspects of intestinal organoids in IBD, such as their potential applications, viability, cell renewal ability, and barrier functionality are highlighted. We also discuss the advances, limitations, and prospects of intestinal organoids for precision medicine.
Conclusion
The latest strides made in research about intestinal organoids help elucidate intricate aspects of IBD pathogenesis, and pave the prospective avenues for novel therapeutic interventions.




Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- IBD:
-
Inflammatory bowel disease
- UC:
-
Ulcerative colitis
- CD:
-
Crohn's disease
- IEC:
-
Intestinal epithelial cell
- 3D:
-
Three-dimensional
- IELs:
-
Intraepithelial lymphocytes
- REF-3-γ:
-
Regenerating family member 3γ
- MUC2:
-
Mucin 2
- TJ:
-
Tight junction
- Gna12:
-
Guanine nucleotide-binding protein subunit-12
- ZO-1:
-
Zonula occludens-1
- Cldn2:
-
Claudin2
- ER:
-
Endoplasmic reticulum
- URP:
-
Unfolded protein response
- STAT3:
-
Signal transducer and activator of transcription 3
- NF-κB:
-
Nuclear factor κB
- ATG16L1:
-
Autophagy-related 16 like 1
- IRGM:
-
Immune-realted guanosine triphosphatase M protein
- DCs:
-
Dendritic cells
- TLR2:
-
Toll-like receptor 2
- ILCs:
-
Innate lymphoid cells
- TNF:
-
Tumor necrosis factor
- IFN:
-
Interferon
- RORC:
-
RAR-related orphan receptor C
- AHR:
-
Aryl hydrocarbon receptor
- MAdCAM-1:
-
Mucosal addressin cell adhesion molecule-1
- Th cells:
-
T helper cells
- Treg:
-
Regulatory T cells
- SCFAs:
-
Short-chain fatty acids
- IL-22BP:
-
Interleukin-22 binding protein
- hIL-22:
-
Human interleukin-22
- HIF-1α:
-
Hypoxia-inducible factor-1α
- DSS:
-
Dextran sulfate sodium
- PHD:
-
Prolyl hydroxylases
- GSDMB:
-
Gasdermin B
- PDGF-A:
-
Platelet-derived growth factor A
- FAK:
-
Focal adhesion kinase
- HA:
-
Hyaluronan
- GAGs:
-
Glycosaminoglycans
- ECM:
-
Extracellular matrix
- MMPs:
-
Matrix metalloproteinases
- JAK:
-
Janus kinase
- PTPN2:
-
Protein tyrosine phosphatase non-receptortype-2
- S1PR:
-
Sphingosine 1-phosphate receptors
- GBP-307:
-
Guanylate binding protein 307
- CCR9:
-
C–C chemokine receptor 9
- rhIL-10:
-
Recombinant human interleukin-10
- TGF-β:
-
Transforming growth factor β
- PDE4:
-
Phosphodiesterase 4
- 3′,5′-cAMP:
-
Cyclic adenosine monophosphate
- MSCs:
-
Mesenchymal stem cells
- HSCs:
-
Hematopoietic stem cells
- PEI:
-
Polyethylenimine
- MONs:
-
Mesoporous organosilica nanoparticles
- cfDNA:
-
Cell-free DNA
- ROS:
-
Reactive oxygen species
- TRAF6:
-
Tumor necrosis factor receptor-associated factor 6
- IRAK1:
-
Interleukin-1 receptor-associated kinase 1
- TNBS:
-
2,4,6-Trinitrobenzene sulfonic acid
- LATS1:
-
Large tumor suppressor kinase 1
- BAs:
-
Bile acids
- FMT:
-
Fecal microbiota transplantation
- 5-HTP:
-
5-Hydroxytryptoph
- iPSCs:
-
Induced pluripotent stem cells
- ISCs:
-
Intestinal stem cells
- TTC7A:
-
Tetratricopeptide repeatdomain 7A
- SMAD4:
-
Suppressor of mothers against decapentaplegic homolog 4
- MLCK:
-
Myosin lightchain kinase
- PPI:
-
Proton pump inhibitor
- MLC:
-
Phosphor-myosin light chain
- MAPK:
-
Mitogen-activated protein kinase
- CS:
-
Systemic corticosteroids
- ATM:
-
Ataxia-telangiectasia mutated proteins
- YAP1:
-
Yes-associatedprotein1
- SOD3:
-
Superoxide dismutase 3
- MLKL:
-
Mixed lineage kinase domain-like protein
- LRH-1:
-
Liver receptor homolog 1
- NKG2D:
-
Naturalkiller group2, member D
- PBMCs:
-
Peripheral blood mononuclear cells
- TP53:
-
Tumor protein 53
- IGF-1:
-
Insulin-like growth factor-1
- PI3K:
-
Phosphoinositide 3-kinase protein kinase B
- PGE2:
-
Prostaglandin E2
- COX:
-
Cyclooxygenase
- CXCL1:
-
C-X-C motif chemokine1
- EP4:
-
Prostaglandin E2 receptor 4
- ATAC:
-
Assay of transposase accessible chromatin
- TEAD:
-
Transcriptional enhanced associate domain
- LPMCs:
-
Lamina propria mononuclear cells
- UCP4:
-
Uncoupling protein 4
- PBAs:
-
Primary bile acids
- SBAs:
-
Secondary bile acids
- TGR5:
-
Takeda G protein-coupled receptor 5
- I3C:
-
Indole-3-carbin
References
Collaborators GBDIBD. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30.
Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Primers. 2020;6(1):74.
Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–36.
Yoo JH, Donowitz M. Intestinal enteroids/organoids: A novel platform for drug discovery in inflammatory bowel diseases. World J Gastroenterol. 2019;25(30):4125–47.
Mehandru S, Colombel JF. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat Rev Gastroenterol Hepatol. 2021;18(2):83–4.
Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–8.
Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56(1):61–72.
Weber CR, Nalle SC, Tretiakova M, Rubin DT, Turner JR. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest. 2008;88(10):1110–20.
Van Klinken BJ, Van der Wal JW, Einerhand AW, Büller HA, Dekker J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut. 1999;44(3):387–93.
Krug SM, Bojarski C, Fromm A, Lee IM, Dames P, Richter JF, et al. Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 2018;11(2):345–56.
Kaser A, Lee A-H, Franke A, Glickman JN, Zeissig S, Tilg H, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134(5):743–56.
Willson TA, Jurickova I, Collins M, Denson LA. Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm Bowel Dis. 2013;19(3):512–25.
Benjamin JL, Sumpter R, Levine B, Hooper LV. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe. 2013;13(6):723–34.
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24.
Hart AL, Al-Hassi HO, Rigby RJ, Bell SJ, Emmanuel AV, Knight SC, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129(1):50–65.
Bain CC, Mowat AM. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260(1):102–17.
de Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27.
Qiu J, Guo X, Chen Z-ME, He L, Sonnenberg GF, Artis D, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39(2):386–99.
Qiu J, Heller JJ, Guo X, Chen Z-ME, Fish K, Fu Y-X, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104.
Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14(3):221–9.
Geremia A, Arancibia-Cárcamo CV, Fleming MPP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208(6):1127–33.
Lamb CA, O’Byrne S, Keir ME, Butcher EC. Gut-selective integrin-targeted therapies for inflammatory bowel disease. J Crohns Colitis. 2018;22(12):S653–68.
Duijvestein M, D’Haens GR. Rational and clinical development of the anti-MAdCAM monoclonal antibody for the treatment of IBD. Expert Opin Biol Ther. 2019;19(4):361–6.
Uhlig HH, Powrie F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu Rev Immunol. 2018;36:755–81.
Fantini MC, Rizzo A, Fina D, Caruso R, Sarra M, Stolfi C, et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology. 2009;136(4):1308–16.
Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23(3):279–87.
Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, Strong SA, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157(3):1261–70.
Pascal V, Pozuelo M, Borruel N, Casellas F, Campos D, Santiago A, et al. A microbial signature for Crohn’s disease. Gut. 2017;66(5):813–22.
Palmela C, Chevarin C, Xu Z, Torres J, Sevrin G, Hirten R, et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut. 2018;67(3):574–87.
Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780–5.
Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–83.
Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, van den Bogaerde J, Samuel D, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389(10075):1218–28.
Schulthess J, Pandey S, Capitani M, Rue-Albrecht KC, Arnold I, Franchini F, et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity. 2019;50(2):432–45.
Imai T, Inoue R, Kawada Y, Morita Y, Inatomi O, Nishida A, et al. Characterization of fungal dysbiosis in Japanese patients with inflammatory bowel disease. J Gastroenterol. 2019;54(2):149–59.
Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160(3):447–60.
Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7(1):68–77.
Mizoguchi A, Yano A, Himuro H, Ezaki Y, Sadanaga T, Mizoguchi E. Clinical importance of IL-22 cascade in IBD. J Gastroenterol. 2018;53(4):465–74.
Pelczar P, Witkowski M, Perez LG, Kempski J, Hammel AG, Brockmann L, et al. A pathogenic role for T cell-derived IL-22BP in inflammatory bowel disease. Science. 2016;354(6310):358–62.
Zhou C, Li L, Li T, Sun L, Yin J, Guan H, et al. SCFAs induce autophagy in intestinal epithelial cells and relieve colitis by stabilizing HIF-1α. J Mol Med (Berl). 2020;98(8):1189–202.
Danese S, Levesque BG, Feagan BG, Jucov A, Bhandari BR, Pai RK, et al. Randomised clinical trial: a phase 1b study of GB004, an oral HIF-1α stabiliser, for treatment of ulcerative colitis. Aliment Pharmacol Ther. 2022;55(4):401–11.
Rana N, Privitera G, Kondolf HC, Bulek K, Lechuga S, De Salvo C, et al. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell. 2022;185(2):283-298.e217.
Aslam MN, McClintock SD, Attili D, Pandya S, Rehman H, Nadeem DM, et al. Ulcerative colitis-derived colonoid culture: a multi-mineral-approach to improve barrier protein expression. Front Cell Dev Biol. 2020;8:577221.
He C, Deng J, Hu X, Zhou S, Wu J, Xiao D, et al. Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 2019;10(2):1235–42.
Lin Y, Li B, Yang X, Liu T, Shi T, Deng B, et al. Non-hematopoietic STAT6 induces epithelial tight junction dysfunction and promotes intestinal inflammation and tumorigenesis. Mucosal Immunol. 2019;12(6):1304–15.
Dong L, Xie J, Wang Y, Jiang H, Chen K, Li D, et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat Commun. 2022;13(1):4804.
Kotla NG, Isa ILM, Rasala S, Demir S, Singh R, Baby BV, et al. Modulation of gut barrier functions in ulcerative colitis by hyaluronic acid system. Adv Sci (Weinh). 2022;9(4):e2103189.
Pu Y, Fan X, Zhang Z, Guo Z, Pan Q, Gao W, et al. Harnessing polymer-derived drug delivery systems for combating inflammatory bowel disease. J Control Release. 2023;354:1–18.
Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, et al. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144(5):989-1000.e1006.
You YD, Deng WH, Guo WY, Zhao L, Mei FC, Hong YP, et al. 4-Phenylbutyric acid attenuates endoplasmic reticulum stress-mediated intestinal epithelial cell apoptosis in rats with severe acute pancreatitis. Dig Dis Sci. 2019;64(6):1535–47.
Liang X, Xie J, Liu H, Zhao R, Zhang W, Wang H, et al. STIM1 deficiency in intestinal epithelium attenuates colonic inflammation and tumorigenesis by reducing ER stress of goblet cells. Cell Mol Gastroenterol Hepatol. 2022;14(1):193–217.
Sandborn WJ, Bhandari BR, Randall C, Younes ZH, Romanczyk T, Xin Y, et al. Andecaliximab [Anti-matrix metalloproteinase-9] induction therapy for ulcerative colitis: a randomised, double-Blind, placebo-controlled, phase 2/3 study in patients with moderate to severe disease. J Crohns Colitis. 2018;12(9):1021–9.
Schreiber S, Siegel CA, Friedenberg KA, Younes ZH, Seidler U, Bhandari BR, et al. A phase 2, randomized, placebo-controlled study evaluating matrix metalloproteinase-9 inhibitor, Andecaliximab, in patients with moderately to severely active Crohn’s disease. J Crohns Colitis. 2018;12(9):1014–20.
Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14(5):269–78.
Casanova MJ, Chaparro M, Mínguez M, Ricart E, Taxonera C, García-López S, et al. Effectiveness and safety of the sequential use of a second and third anti-TNF agent in patients with inflammatory bowel disease: results from the eneida registry. Inflamm Bowel Dis. 2020;26(4):606–16.
Hedl M, Proctor DD, Abraham C. JAK2 disease-risk variants are gain of function and JAK signaling threshold determines innate receptor-induced proinflammatory cytokine secretion in macrophages. J Immunol. 2016;197(9):3695–704.
Núñez P, Quera R, Yarur AJ. Safety of janus kinase inhibitors in inflammatory bowel diseases. Drugs. 2023;83(4):299–314.
Spalinger MR, Sayoc-Becerra A, Ordookhanian C, Canale V, Santos AN, King SJ, et al. The JAK inhibitor Tofacitinib rescues intestinal barrier defects caused by disrupted epithelial-macrophage interactions. J Crohns Colitis. 2021;15(3):471–84.
Deepak P, Alayo QA, Khatiwada A, Lin B, Fenster M, Dimopoulos C, et al. Safety of Tofacitinib in a real-world cohort of patients with ulcerative colitis. Clinical Gastroenterol. 2021;19(8):1592-1601.e1593.
Burnett Z, Werner BC. Risk factors, management, and prognosis of brachial plexopathy following reverse total shoulder arthroplasty. Orthop Clin North Am. 2022;53(2):215–21.
Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330(3):864–75.
Peyrin-Biroulet L, Hart A, Bossuyt P, Long M, Allez M, Juillerat P, et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): a phase 3, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2022;7(2):128–40.
MacDonald JK, McDonald JW. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007;1:CD006097.
Sandborn WJ, Feagan BG, Hanauer S, Vermeire S, Ghosh S, Liu WJ, et al. Long-term efficacy and safety of ozanimod in moderately to severely active ulcerative colitis: results from the open-label extension of the randomized, phase 2 TOUCHSTONE study. J Crohns Colitis. 2021;15(7):1120–9.
Sandborn WJ, Peyrin-Biroulet L, Zhang J, Chiorean M, Vermeire S, Lee SD, et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):550–61.
Correction for Yu et al (2022) Structural insights into sphingosine-1-phosphate receptor activation. Proc Natl Acad Sci USA 119(34): e2209949119
Keshav S, Vaňásek T, Niv Y, Petryka R, Howaldt S, Bafutto M, et al. A randomized controlled trial of the efficacy and safety of CCX282-B, an orally-administered blocker of chemokine receptor CCR9, for patients with Crohn’s disease. PLoS ONE. 2013;8(3):e60094.
Feagan BG, Sandborn WJ, D’Haens G, Lee SD, Allez M, Fedorak RN, et al. Randomised clinical trial: vercirnon, an oral CCR9 antagonist, vs. placebo as induction therapy in active Crohn’s disease. Aliment Pharmacol Ther. 2015;42(10):1170–81.
Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6(5):583–8.
Danese S, Vermeire S, Hellstern P, Panaccione R, Rogler G, Fraser G, et al. Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn’s disease (ANDANTE I and II). Gut. 2019;68(1):40–8.
Schreiber S, Aden K, Bernardes JP, Conrad C, Tran F, Höper H, et al. Therapeutic interleukin-6 trans-signaling inhibition by Olamkicept (sgp130Fc) in patients with active inflammatory bowel disease. Gastroenterology. 2021;160(7):2354-2366.e2311.
Schreiber S, Fedorak RN, Nielsen OH, Wild G, Williams CN, Nikolaus S, et al. Safety and efficacy of recombinant human interleukin 10 in chronic active Crohn’s disease Crohn’s Disease IL-10 Cooperative Study Group. Gastroenterology. 2000;119(6):1461–72.
Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology. 1999;116(5):1107–14.
Clough JN, Omer OS, Tasker S, Lord GM, Irving PM. Regulatory T-cell therapy in Crohn’s disease: challenges and advances. Gut. 2020;69(5):942–52.
Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143(5):1207-1217.e1202.
Voskens CJ, Stoica D, Roessner S, Vitali F, Zundler S, Rosenberg M, et al. Safety and tolerability of a single infusion of autologous ex vivo expanded regulatory T cells in adults with ulcerative colitis (ER-TREG 01): protocol of a phase 1, open-label, fast-track dose-escalation clinical trial. BMJ Open. 2021;11(12):e049208.
Danese S, Neurath MF, Kopoń A, Zakko SF, Simmons TC, Fogel R, et al. Effects of Apremilast, an oral inhibitor of phosphodiesterase 4, in a randomized trial of patients with active ulcerative colitis. Clin Gastroenterol Hepatol. 2020;18(11):2526-2534.e2529.
Liu H, Wang Q, Huang Y, Deng J, Xie X, Zhu J, et al. Discovery of novel PDE4 inhibitors targeting the M-pocket from natural mangostanin with improved safety for the treatment of inflammatory bowel diseases. Eur J Med Chem. 2022;242:114631.
Barnhoorn MC, Wasser M, Roelofs H, Maljaars PWJ, Molendijk I, Bonsing BA, et al. Long-term evaluation of allogeneic bone marrow-derived mesenchymal stromal cell therapy for Crohn’s disease perianal fistulas. J Crohns Colitis. 2020;14(1):64–70.
Brierley CK, Castilla-Llorente C, Labopin M, Badoglio M, Rovira M, Ricart E, et al. Autologous haematopoietic stem cell transplantation for Crohn’s disease: a retrospective survey of long-term outcomes from the European society for blood and marrow transplantation. J Crohns Colitis. 2018;12(9):1097–103.
Chen J, Huang J, Shi J, Li M, Zhao E, Li G, et al. Nestin+ Peyer’s patch resident MSCs enhance healing of inflammatory bowel disease through IL-22-mediated intestinal epithelial repair. Cell Prolif. 2023;56(2):e13363.
Zhang Y, Wang T, Sun M, Song Y, Huang X, Zhang S, et al. Advanced nanomedicine: redefining therapeutic paradigms for inflammatory bowel disease. Adv Healthc Mater. 2023;12:e2300069.
Shi C, Dawulieti J, Shi F, Yang C, Qin Q, Shi T, et al. A nanoparticulate dual scavenger for targeted therapy of inflammatory bowel disease. Sci Adv. 2022;8(4):2372.
Wang C, Xu M, Fan Q, Li C, Zhou X. Therapeutic potential of exosome-based personalized delivery platform in chronic inflammatory diseases. Asian J Pharm Sci. 2023;18(1):100772.
Wu H, Fan H, Shou Z, Xu M, Chen Q, Ai C, et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204–12.
Deng F, Yan J, Lu J, Luo M, Xia P, Liu S, et al. M2 macrophage-derived exosomal miR-590-3p attenuates DSS-induced mucosal damage and promotes epithelial repair via the LATS1/YAP/ β-Catenin signalling axis. J Crohns Colitis. 2021;15(4):665–77.
Shan Y, Lee M, Chang EB. The gut microbiome and inflammatory bowel diseases. Annu Rev Med. 2022;73:455–68.
Martyniak A, Medyńska-Przęczek A, Wędrychowicz A, Skoczeń S, Tomasik PJ. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules. 2021;11:12.
Fedorak RN, Feagan BG, Hotte N, Leddin D, Dieleman LA, Petrunia DM, et al. The probiotic VSL#3 has anti-inflammatory effects and could reduce endoscopic recurrence after surgery for Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13(5):928-935.e922.
Furrie E, Macfarlane S, Kennedy A, Cummings JH, Walsh SV, O’Neil DA, et al. Synbiotic therapy (Bifidobacterium longum/Synergy 1) initiates resolution of inflammation in patients with active ulcerative colitis: a randomised controlled pilot trial. Gut. 2005;54(2):242–9.
Ooijevaar RE, Terveer EM, Verspaget HW, Kuijper EJ, Keller JJ. Clinical application and potential of fecal microbiota transplantation. Annu Rev Med. 2019;70:335–51.
Moayyedi P, Surette MG, Kim PT, Libertucci J, Wolfe M, Onischi C, et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology. 2015;149(1):102-109 e106.
Kump PK, Grochenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19(10):2155–65.
Sokol H, Landman C, Seksik P, Berard L, Montil M, Nion-Larmurier I, et al. Fecal microbiota transplantation to maintain remission in Crohn’s disease: a pilot randomized controlled study. Microbiome. 2020;8(1):12.
Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277.
Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TW, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–62.
Cai J, Sun L, Gonzalez FJ. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe. 2022;30(3):289–300.
Chen L, Jiao T, Liu W, Luo Y, Wang J, Guo X, et al. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell. 2022;29(9):1366–81.
Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge J, et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153(6):1504-1516.e1502.
Truyens M, Lobatón T, Ferrante M, Bossuyt P, Vermeire S, Pouillon L, et al. Effect of 5-Hydroxytryptophan on fatigue in quiescent inflammatory bowel disease: a randomized controlled trial. Gastroenterology. 2022;163(5):1294-1305.e1293.
Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19(11):671–87.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–5.
Dedhia PH, Bertaux-Skeirik N, Zavros Y, Spence JR. Organoid models of human gastrointestinal development and disease. Gastroenterology. 2016;150(5):1098–112.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–9.
Kim MB, Hwangbo S, Jang S, Jo YK. Bioengineered Co-culture of organoids to recapitulate host–microbe interactions. Mater Today Bio. 2022;16:100345.
Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141(5):1762–72.
Noben M, Verstockt B, de Bruyn M, Hendriks N, Van Assche G, Vermeire S, et al. Epithelial organoid cultures from patients with ulcerative colitis and Crohn’s disease: a truly long-term model to study the molecular basis for inflammatory bowel disease? Gut. 2017;66(12):2193–5.
Howell KJ, Kraiczy J, Nayak KM, Gasparetto M, Ross A, Lee C, et al. DNA methylation and transcription patterns in intestinal epithelial cells from pediatric patients with inflammatory bowel diseases differentiate disease subtypes and associate with outcome. Gastroenterology. 2018;154(3):585–98.
Boye TL, Steenholdt C, Jensen KB, Nielsen OH. Molecular manipulations and intestinal stem cell-derived organoids in inflammatory bowel disease. Stem Cells. 2022;40(5):447–57.
Stewart AS, Schaaf CR, Luff JA, Freund JM, Becker TC, Tufts SR, et al. HOPX(+) injury-resistant intestinal stem cells drive epithelial recovery after severe intestinal ischemia. Am J Physiol Gastrointest Liver Physiol. 2021;321(5):G588–602.
Al-Lamki RS, Bradley JR, Pober JS. Human organ culture: updating the approach to bridge the gap from in vitro to in vivo in inflammation, cancer, and stem cell biology. Front Med (Lausanne). 2017;4:148.
Kozuka K, He Y, Koo-McCoy S, Kumaraswamy P, Nie B, Shaw K, et al. Development and characterization of a human and mouse intestinal epithelial cell monolayer platform. Stem Cell Reports. 2017;9(6):1976–90.
Jardine S, Anderson S, Babcock S, Leung G, Pan J, Dhingani N, et al. Drug screen identifies leflunomide for treatment of inflammatory bowel disease caused by TTC7A deficiency. Gastroenterology. 2020;158(4):1000–15.
Buckley A, Turner JR. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harb Perspect Biol. 2018;10:1.
Xu P, Elizalde M, Masclee A, Pierik M, Jonkers D. Corticosteroid enhances epithelial barrier function in intestinal organoids derived from patients with Crohn’s disease. J Mol Med (Berl). 2021;99(6):805–15.
Kim MR, Cho SY, Lee HJ, Kim JY, Nguyen UTT, Ha NM, et al. Schisandrin C improves leaky gut conditions in intestinal cell monolayer, organoid, and nematode models by increasing tight junction protein expression. Phytomedicine. 2022;103:154209.
Marincola Smith P, Choksi YA, Markham NO, Hanna DN, Zi J, Weaver CJ, et al. Colon epithelial cell TGFβ signaling modulates the expression of tight junction proteins and barrier function in mice. Am J Physiol Gastrointest Liver Physiol. 2021;320(6):G936-g957.
Martínez-Sánchez LDC, Ngo PA, Pradhan R, Becker LS, Boehringer D, Soteriou D, et al. Epithelial RAC1-dependent cytoskeleton dynamics controls cell mechanics, cell shedding and barrier integrity in intestinal inflammation. Gut. 2023;72(2):275–94.
Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809.
Nighot M, Liao PL, Morris N, McCarthy D, Dharmaprakash V, Khan IU, et al. Long term use of proton pump inhibitor disrupts intestinal tight junction barrier and exaggerates experimental colitis. J Crohns Colitis. 2022. https://doi.org/10.1093/ecco-jcc/jjac168.
Shah R, Richardson P, Yu H, Kramer J, Hou JK. Gastric acid suppression is associated with an increased risk of adverse outcomes in inflammatory bowel disease. Digestion. 2017;95(3):188–93.
Dubois-Camacho K, Ottum PA, Franco-Munoz D, De la Fuente M, Torres-Riquelme A, Diaz-Jimenez D, et al. Glucocorticosteroid therapy in inflammatory bowel diseases: from clinical practice to molecular biology. World J Gastroenterol. 2017;23(36):6628–38.
Reyes EA, Castillo-Azofeifa D, Rispal J, Wald T, Zwick RK, Palikuqi B, et al. Epithelial TNF controls cell differentiation and CFTR activity to maintain intestinal mucin homeostasis. J Clin Invest. 2023. https://doi.org/10.1172/JCI163591.
d’Aldebert E, Quaranta M, Sebert M, Bonnet D, Kirzin S, Portier G, et al. Characterization of human colon organoids from inflammatory bowel disease patients. Front Cell Dev Biol. 2020;8:363.
Lee C, Hong SN, Kim ER, Chang DK, Kim YH. Epithelial regeneration ability of Crohn’s disease assessed using patient-derived intestinal organoids. Int J Mol Sci. 2021;22(11):6013.
Qian J, Zhao W, Miao X, Li L, Zhang D. Sam68 modulates apoptosis of intestinal epithelial cells via mediating NF-kappaB activation in ulcerative colitis. Mol Immunol. 2016;75:48–59.
Goodman WA, Basavarajappa SC, Liu AR, Rodriguez FDS, Mathes T, Ramakrishnan P. Sam68 contributes to intestinal inflammation in experimental and human colitis. Cell Mol Life Sci. 2021;78(23):7635–48.
Tak LJ, Kim HY, Ham WK, Agrahari G, Seo Y, Yang JW, et al. Superoxide dismutase 3-transduced mesenchymal stem cells preserve epithelial tight junction barrier in murine colitis and attenuate inflammatory damage in epithelial organoids. Int J Mol Sci. 2021;22(12):6431.
Lee C, An M, Joung JG, Park WY, Chang DK, Kim YH, et al. TNFα Induces LGR5+ stem cell dysfunction in patients with Crohn’s disease. Cell Mol Gastroenterol Hepatol. 2022;13(3):789–808.
Bayrer JR, Wang H, Nattiv R, Suzawa M, Escusa HS, Fletterick RJ, et al. LRH-1 mitigates intestinal inflammatory disease by maintaining epithelial homeostasis and cell survival. Nat Commun. 2018;9(1):4055.
Hammoudi N, Hamoudi S, Bonnereau J, Bottois H, Pérez K, Bezault M, et al. Autologous organoid co-culture model reveals T cell-driven epithelial cell death in Crohn’s Disease. Front Immunol. 2022;13:1008456.
Angus HC, Urbano PC, Laws GA, Fan S, Gadeock S, Schultz M, et al. An autologous colonic organoid-derived monolayer model to study immune: bacterial interactions in Crohn’s disease patients. Clin Transl Immunol. 2022;11(8):e1407.
Watanabe S, Hibiya S, Katsukura N, Kitagawa S, Sato A, Okamoto R, et al. Importance of telomere shortening in the pathogenesis of ulcerative colitis: a new treatment from the aspect of telomeres in intestinal epithelial cells. J Crohns Colitis. 2022;16(1):109–21.
Chakravarti D, Lee R, Multani AS, Santoni A, Keith Z, Hsu WH, et al. Telomere dysfunction instigates inflammation in inflammatory bowel disease. Proc Natl Acad Sci U S A. 2021;118(29):e2024853118.
Chakravarti D, Hu B, Mao X, Rashid A, Li J, Li J, et al. Telomere dysfunction activates YAP1 to drive tissue inflammation. Nat Commun. 2020;11(1):4766.
Yu H, Yang X, Xiao X, Xu M, Yang Y, Xue C, et al. Human adipose mesenchymal stem cell-derived exosomes protect mice from DSS-induced inflammatory bowel disease by promoting intestinal-stem-cell and epithelial regeneration. Aging Dis. 2021;12(6):1423–37.
Liang X, Li C, Song J, Liu A, Wang C, Wang W, et al. HucMSC-Exo promote mucosal healing in experimental colitis by accelerating intestinal stem cells and epithelium regeneration via Wnt signaling pathway. Int J Nanomed. 2023;18:2799–818.
Xu J, Wang X, Chen J, Chen S, Li Z, Liu H, et al. Embryonic stem cell-derived mesenchymal stem cells promote colon epithelial integrity and regeneration by elevating circulating IGF-1 in colitis mice. Theranostics. 2020;10(26):12204–22.
Carulli AJ, Keeley TM, Demitrack ES, Chung J, Maillard I, Samuelson LC. Notch receptor regulation of intestinal stem cell homeostasis and crypt regeneration. Dev Biol. 2015;402(1):98–108.
Na YR, Jung D, Stakenborg M, Jang H, Gu GJ, Jeong MR, et al. Prostaglandin E(2) receptor PTGER4-expressing macrophages promote intestinal epithelial barrier regeneration upon inflammation. Gut. 2021;70(12):2249–60.
Wang Y, Chiang IL, Ohara TE, Fujii S, Cheng J, Muegge BD, et al. Long-term culture captures injury-repair cycles of colonic stem cells. Cell. 2019;179:5:1144–1159 e1115.
Kobayashi S, Ogasawara N, Watanabe S, Yoneyama Y, Kirino S, Hiraguri Y, et al. Collagen type I-mediated mechanotransduction controls epithelial cell fate conversion during intestinal inflammation. Inflamm Regen. 2022;42(1):49.
Powell N, Pantazi E, Pavlidis P, Tsakmaki A, Li K, Yang F, et al. Interleukin-22 orchestrates a pathological endoplasmic reticulum stress response transcriptional programme in colonic epithelial cells. Gut. 2020;69(3):578–90.
Schmechel S, Konrad A, Diegelmann J, Glas J, Wetzke M, Paschos E, et al. Linking genetic susceptibility to Crohn’s disease with Th17 cell function: IL-22 serum levels are increased in Crohn’s disease and correlate with disease activity and IL23R genotype status. Inflamm Bowel Dis. 2008;14(2):204–12.
He GW, Lin L, DeMartino J, Zheng X, Staliarova N, Dayton T, et al. Optimized human intestinal organoid model reveals interleukin-22-dependency of paneth cell formation. Cell Stem Cell. 2022;29(9):1333-1345 e1336.
Tan C, Hong G, Wang Z, Duan C, Hou L, Wu J, et al. Promoting effect of L-Fucose on the regeneration of intestinal stem cells through AHR/IL-22 pathway of intestinal lamina propria monocytes. Nutrients. 2022;14(22):4789.
Deleu S, Arnauts K, Deprez L, Machiels K, Ferrante M, Huys GRB, et al. High acetate concentration protects intestinal barrier and exerts anti-inflammatory effects in organoid-derived epithelial monolayer cultures from patients with ulcerative colitis. Int J Mol Sci. 2023;24(1):768.
Ye J, Haskey N, Dadlani H, Zubaidi H, Barnett JA, Ghosh S, et al. Deletion of mucin 2 induces colitis with concomitant metabolic abnormalities in mice. Am J Physiol Gastrointest Liver Physiol. 2021;320(5):G791–803.
Jurickova I, Bonkowski E, Angerman E, Novak E, Huron A, Akers G, et al. Eicosatetraynoic acid and butyrate regulate human intestinal organoid mitochondrial and extracellular matrix pathways implicated in Crohn’s disease strictures. Inflamm Bowel Dis. 2022;28(7):988–1003.
Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, et al. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006;8(3):389–414.
Gallagher K, Catesson A, Griffin JL, Holmes E, Williams HRT. Metabolomic analysis in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2021;15(5):813–26.
Sorrentino G, Perino A, Yildiz E, El Alam G, Bou Sleiman M, Gioiello A, et al. Bile acids signal via TGR5 to activate intestinal stem cells and epithelial regeneration. Gastroenterology. 2020;159(3):956-968 e958.
Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–37.
Park JH, Lee JM, Lee EJ, Hwang WB, Kim DJ. Indole-3-carbinol promotes goblet-cell differentiation regulating Wnt and Notch signaling pathways AhR-dependently. Mol Cells. 2018;41(4):290–300.
Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294(5549):2155–8.
Giri R, Hoedt EC, Khushi S, Salim AA, Bergot AS, Schreiber V, et al. Secreted NF-κB suppressive microbial metabolites modulate gut inflammation. Cell Rep. 2022;39(2):110646.
Quevrain E, Maubert MA, Michon C, Chain F, Marquant R, Tailhades J, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in Crohn’s disease. Gut. 2016;65(3):415–25.
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18(4):618–23.
Morris SA, Cahan P, Li H, Zhao AM, San Roman AK, Shivdasani RA, et al. Dissecting engineered cell types and enhancing cell fate conversion via Cell Net. Cell. 2014;158(4):889–902.
Fukuda M, Mizutani T, Mochizuki W, Matsumoto T, Nozaki K, Sakamaki Y, et al. Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 2014;28(16):1752–7.
Watanabe S, Kobayashi S, Ogasawara N, Okamoto R, Nakamura T, Watanabe M, et al. Transplantation of intestinal organoids into a mouse model of colitis. Nat Protoc. 2022;17(3):649–71.
Valatas V, Bamias G, Kolios G. Experimental colitis models: Insights into the pathogenesis of inflammatory bowel disease and translational issues. Eur J Pharmacol. 2015;759:253–64.
Voskens C, Stoica D, Rosenberg M, Vitali F, Zundler S, Ganslmayer M, et al. Autologous regulatory T-cell transfer in refractory ulcerative colitis with concomitant primary sclerosing cholangitis. Gut. 2023;72(1):49–53.
Sands BE, Peyrin-Biroulet L, Kierkus J, Higgins PDR, Fischer M, Jairath V, et al. Efficacy and safety of Mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022;162(2):495–508.
Funding
This study was supported by the Science and Technology Plan Project of Wenzhou, China (Grant Numbers Y20220045, Y20220389), Zhejiang Province Natural Science Foundation of China (Grant Number LTGY23H100001), National Natural Science Foundation of China (Grant Number 81901660), and Key Laboratory of Clinical Laboratory Diagnosis and Translational Research of Zhejiang Province (2022E10022).
Author information
Authors and Affiliations
Contributions
CH and SG conceived this Review. LK, SH, SC, and AZ searched the literature and drafted the manuscript. SC and SH completed the drawing of the Figures. SG edited the manuscript, JY and CH revised the manuscript. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare there are no competing interests.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, L., Chen, S., Huang, S. et al. Challenges and opportunities in inflammatory bowel disease: from current therapeutic strategies to organoid-based models. Inflamm. Res. 73, 541–562 (2024). https://doi.org/10.1007/s00011-024-01854-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-024-01854-z