Abstract
Background
Osteoarthritis (OA) is one of degenerative-related arthritis, which can be aggravated by low-grade synovitis. It is known that arachidonic acid (AA) dysmetabolism brings OA synovitis. However, the impact of synovial AA metabolism pathway (AMP) related genes on OA remains uncovered.
Methods
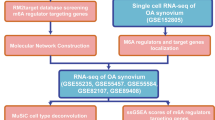
Here, we conducted a comprehensive analysis to explore the impact of AA metabolism genes in OA synovium. We obtained transcriptome expression profiles from three raw datasets related to OA synovium (GSE12021, GSE29746, GSE55235) and identified the hub genes of AA metabolism pathways (AMP) in OA synovium. An OA occurrence diagnostic model was constructed and validated based on the identified hub genes. Then, we explored the correlation between hub gene expression and the immune-related module using CIBERSORT and MCP-counter analysis. The unsupervised consensus clustering analysis and weighted correlation network analysis (WGCNA) were utilized to identify robust clusters of identified genes in each cohort. Moreover, the interaction between the hub genes of AMP and immune cells was elucidated through single-cell RNA (scRNA) analysis by scRNA sequencing data from GSE152815.
Results
We found that the expression of AMP-related genes was up-regulated in OA synovium, and seven hub genes (LTC4S, PTGS2, PTGS1, MAPKAPK2, CBR1, PTGDS, and CYP2U1) were identified. The diagnostic model that combined the identified hub genes showed great clinical validity in diagnosing OA (AUC = 0.979). Moreover, significant associations were noticed between the hub genes' expression, immune cell infiltration, and inflammatory cytokine levels. The 30 OA patients were randomized and clustered into three groups using WGCNA analysis based on the hub genes, and diverse immune status was found in different clusters. Of interest, older patients were more likely to be classified into a cluster with higher levels of inflammatory cytokines IL-6 and less infiltration of immune cells. Based on the scRNA-sequencing data, we found that the hub genes had relatively higher expression in macrophages and B cells than other immune cells. Moreover, inflammation-related pathways were significantly enriched in macrophages.
Conclusion
These results suggest that AMP-related genes are closely involved in alterations of OA synovial inflammation. The transcriptional level of hub genes could serve as a potential diagnostic marker for OA.










Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding authors upon reasonable request.
References
Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12(11):632–44.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum-Us. 2012;64(6):1697–707.
Hunter DJ, March L, Chew M. Osteoarthritis in 2020 and beyond–Authors’ reply. The Lancet. 2021;397:10279.
Thomas E, Peat G, Croft P. Defining and mapping the person with osteoarthritis for population studies and public health. Rheumatology. 2014;53(2):338–45.
Nelson AE, Allen KD, Golightly YM, Goode AR, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: The Chronic Osteoarthritis Management Initiative of the US Bone and Joint Initiative. Semin Arthritis Rheu. 2014;43(6):701–12.
Roos EM, Arden NK. Strategies for the prevention of knee osteoarthritis. Nat Rev Rheumatol. 2016;12(2):92–101.
Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
Recht MP, Resnick D. MR imaging of articular cartilage: current status and future directions. AJR Am J Roentgenol. 1994;163(2):283–90.
Chou CH, Gibson J, Attarian DE, Haraden C, Yohn CB, Laberge RM, Gregory S, Kraus VB. Profiling Human Chondrocytes and Synoviocytes Using Single Cell Rna Sequencing Identifies Cell Diversity in the Pathogenesis of Osteoarthritis in the Joint Organ. Osteoarthr Cartilage. 2019;27:S27–S27.
Wang X, Chen T, Liang W, Fan T, Zhu Z, Cao P, Ruan G, Zhang Y, Chen S, Wang Q, et al. Synovitis mediates the association between bone marrow lesions and knee pain in osteoarthritis: data from the Foundation for the National Institute of Health (FNIH) Osteoarthritis Biomarkers Consortium. Osteoarthritis Cartilage. 2022;30(9):1270–7.
Wyatt LA, Mapp PI, Moreton BJ, Wilson D, Hill R, Ferguson E, Scammell BE, Walsh DA. Histopathological Subgroups in Knee Osteoarthritis. Osteoarthr Cartilage. 2016;24:S386–S386.
Roemer FW, Guermazi A, Felson DT, Niu JB, Nevitt MC, Crema MD, Lynch JA, Lewis CE, Torner J, Zhang YQ. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70(10):1804–9.
Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–35.
Philpott HT, Birmingham TB, Pinto R, Primeau CA, Arsenault D, Lanting BA, Zhu Y, Appleton CT. Synovitis is associated with constant pain in knee osteoarthritis: a cross-sectional study of OMERACT knee ultrasound scores. J Rheumatol. 2022;49(1):89–97.
Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13(5):302–11.
Gonzalez-Perilli L, Prolo C, Alvarez MN. Arachidonic acid and nitroarachidonic: effects on NADPH oxidase activity. Adv Exp Med Biol. 2019;1127:85–95.
Van de Vyver A, Clockaerts S, van de Lest CHA, Wei W, Verhaar J, Van Osch G, Bastiaansen-Jenniskens YM. Synovial fluid fatty acid profiles differ between osteoarthritis and healthy patients. Cartilage. 2020;11(4):473–8.
Seo MJ, Oh DK. Prostaglandin synthases: molecular characterization and involvement in prostaglandin biosynthesis. Prog Lipid Res. 2017;66:50–68.
Ye ZD, Shen Y, Jin K, Qiu JT, Hu B, Jadhav RR, Sheth K, Weyand CM, Goronzy JJ. Arachidonic acid-regulated calcium signaling in T cells from patients with rheumatoid arthritis promotes synovial inflammation. Nat Commun. 2021. https://doi.org/10.1038/s41467-021-21242-z.
Monti S, Tamayo P, Mesirov J, Golub T. Consensus clustering: a resampling-based method for class discovery and visualization of gene expression microarray data. Mach Learn. 2003;52(1–2):91–118.
Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol. 2005. https://doi.org/10.2202/1544-6115.1128.
Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009. https://doi.org/10.1186/1471-2105-10-48.
Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics. 2013. https://doi.org/10.1186/1471-2105-14-7.
Aibar S, Gonzalez-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G, Rambow F, Marine JC, Geurts P, Aerts J, et al. SCENIC: single-cell regulatory network inference and clustering. Nat Methods. 2017;14(11):1083–6.
Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 30. Bioinformatics. 2011;27(12):1739–40.
Zhang H, Cai D, Bai X. Macrophages regulate the progression of osteoarthritis. Osteoarthr Cartilage. 2020;28(5):555–61.
Liu S, Deng Z, Chen K, Jian S, Zhou F, Yang Y, Fu Z, Xie H, Xiong J, Zhu W. Cartilage tissue engineering: From pro-inflammatory and anti-inflammatory cytokines to osteoarthritis treatments (Review). Mol Med Rep. 2022;25(3):615.
Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors. Emerg Concepts Allergy Asthma Immun. 2014;6(4):288–95.
Kanaoka Y, Austen KF. Roles of cysteinyl leukotrienes and their receptors in immune cell-related functions. Adv Immunol. 2019;142:65–84.
Chan MWY, Gomez-Aristizabal A, Mahomed N, Gandhi R, Viswanathan S. A tool for evaluating novel osteoarthritis therapies using multivariate analyses of human cartilage-synovium explant co-culture. Osteoarthr Cartilage. 2022;30(1):147–59.
Jia X, Shao W, Tian S. Berberine alleviates myocardial ischemia-reperfusion injury by inhibiting inflammatory response and oxidative stress: the key function of miR-26b-5p-mediated PTGS2/MAPK signal transduction. Pharm Biol. 2022;60(1):652–63.
Su W, Liu G, Mohajer B, Wang J, Shen A, Zhang W, Liu B, Guermazi A, Gao P, Cao X, et al. Senescent preosteoclast secretome promotes metabolic syndrome associated osteoarthritis through cyclooxygenase. Elife. 2022;2:11.
Tive L. Celecoxib clinical profile. Rheumatology. 2000;39:21–8.
Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartilage. 2019;27(11):1578–89.
Hawkey CJ. COX-1 and COX-2 inhibitors. Best Pract Res Clin Gastroenterol. 2001;15(5):801–20.
Wang CJ, Wang F, Lin F, Duan XH, Bi BN. Naproxen attenuates osteoarthritis progression through inhibiting the expression of prostaglandinl-endoperoxide synthase 1. J Cell Physiol. 2019;234(8):12771–85.
Jones SW, Brockbank SMV, Clements KM, Le Good N, Campbell D, Read SJ, Needham MRC, Newham P. Mitogen-activated protein kinase-activated protein kinase 2 (MK2) modulates key biological pathways associated with OA disease pathology. Osteoarthr Carti. 2009;17(1):124–31.
Miyazawa K, Mori A, Miyata H, Akahane M, Ajisawa Y, Okudaira H. Regulation of interleukin-1beta-induced interleukin-6 gene expression in human fibroblast-like synoviocytes by p38 mitogen-activated protein kinase. J Biol Chem. 1998;273(38):24832–8.
Vitale P, Panella A, Scilimati A. Beyond Structure Toward Therapy. Med Res Rev. 2016;36(4):641–71.
Forrest GL, Gonzalez B. Carbonyl reductase. Chem Biol Interact. 2000;129(1–2):21–40.
Shi SM, Di L. The role of carbonyl reductase 1 in drug discovery and development. Expert Opin Drug Metab Toxicol. 2017;13(8):859–70.
Tak E, Lee S, Lee J, Rashid MA, Kim YW, Park JH, Park WS, Shokat KM, Ha J, Kim SS. Human carbonyl reductase 1 up-regulated by hypoxia renders resistance to apoptosis in hepatocellular carcinoma cells. J Hepatol. 2011;54(2):328–39.
Kraus VB, Blanco FJ, Englund M, Karsdal MA, Lohmander LS. Call for standardized definitions of osteoarthritis and risk stratification for clinical trials and clinical use. Osteoarthr Cartilage. 2015;23(8):1233–41.
Woodell-May JE, Sommerfeld SD. Role of Inflammation and the Immune System in the Progression of Osteoarthritis. J Orthop Res. 2020;38(2):253–7.
Sun H, Zhang Y, Song W, Yin L, Wang G, Yu D, Zhang Q, Yan X, Li S. IgM(+)CD27(+) B cells possessed regulatory function and represented the main source of B cell-derived IL-10 in the synovial fluid of osteoarthritis patients. Hum Immunol. 2019;80(4):263–9.
Hu XY, Ni SJ, Zhao K, Qian J, Duan Y. Bioinformatics-Led discovery of osteoarthritis biomarkers and inflammatory infiltrates. Front Immunol. 2022;13:313.
Sanchez-Lopez E, Coras R, Torres A, Lane NE, Guma M. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–75.
Lin RC, Deng CJ, Li XX, Liu YQ, Zhang M, Qin C, Yao QQ, Wang LM, Wu CT. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics. 2019;9(21):6300–13.
Conaghan PG, Cook AD, Hamilton JA, Tak PP. Therapeutic options for targeting inflammatory osteoarthritis pain. Nat Rev Rheumatol. 2019;15(6):355–63.
Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1966–71.
Aitken D, Laslett LL, Pan F, Haugen IK, Otahal P, Bellamy N, Bird P, Jones G. A randomized double-blind placebo-controlled crossover trial of HUMira (adalimumab) for erosive hand OsteoaRthritis - the HUMOR trial. Osteoarthr Cartilage. 2018;26(7):880–7.
Kloppenburg M, Ramonda R, Bobacz K, Kwok WY, Elewaut D, Huizinga TWJ, Kroon FPB, Punzi L, Smolen JS, Vander Cruyssen B, et al. Etanercept in patients with inflammatory hand osteoarthritis (EHOA): a multicentre, randomized, double-blind, placebo-controlled trial. Ann Rheum Dis. 2018;77(12):1757–64.
Van den Bossche J, Baardman J, Otto NA, van der Velden S, Neele AE, van den Berg SM, Luque-Martin R, Chen HJ, Boshuizen MC, Ahmed M, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17(3):684–96.
Mehana EE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: An updated review. Life Sci. 2019;234:116786.
Lu J, Feng X, Zhang H, Wei Y, Yang Y, Tian Y, Bai L. Maresin-1 suppresses IL-1β-induced MMP-13 secretion by activating the PI3K/AKT pathway and inhibiting the NF-κB pathway in synovioblasts of an osteoarthritis rat model with treadmill exercise. Connect Tissue Res. 2020;62(5):508–18.
Acknowledgements
We thank all the people who offer help for this study. Co-first authors: Bizhi Tu, Run Fang, and Zheng Zhu contributed equally to this paper.
Funding
This study was supported by Grants from Anhui Key Clinical Speciality Construction Project.
Author information
Authors and Affiliations
Contributions
Rende Ning conceived the study idea, revised the manuscript, and provided financial support. BT, RF collected the data and wrote the initial draft. ZZ, CP and GC contributed to the data collection and analysis. All authors approved the final draft of the manuscript. All authors are accountable for all aspects of the work in ensuring related questions' accuracy or integrity. Any parts of the work are appropriately investigated and resolved. NR is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest in this work.
Ethics approval and consent to participate
Ethical approval for our study was granted by The Committee on Medical Ethics of The Third Affiliated Hospital of Anhui Medical University (Reference number 2022 (69)). Since all the data used in the current study was available online, and no individual patient was involved, it could be confirmed we have obtained all the written informed consent.
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.





Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tu, B., Fang, R., Zhu, Z. et al. Comprehensive analysis of arachidonic acid metabolism-related genes in diagnosis and synovial immune in osteoarthritis: based on bulk and single-cell RNA sequencing data. Inflamm. Res. 72, 955–970 (2023). https://doi.org/10.1007/s00011-023-01720-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01720-4