Abstract
Objective and methods
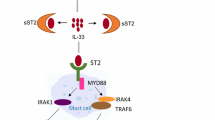
IL-33 is present in endothelial, epithelial, and fibroblast-like cells and released upon cell injury. IL-33 reportedly induces mast-cell degranulation and is involved in various diseases, including allergic diseases. So, IL-33-related diseases seem to overlap with histamine-related diseases. In addition to the release from mast cells, histamine is newly formed by the induction of histidine decarboxylase (HDC). Some inflammatory and/or hematopoietic cytokines (IL-1, IL-3, etc.) are known to induce HDC, and the histamine produced by HDC induction is released without storage. We examined the involvement of HDC and histamine in the effects of IL-33.
Results
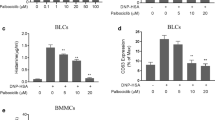
A single intraperitoneal injection of IL-33 into mice induced HDC directly and/or via other cytokines (including IL-5) within a few hours in various tissues, particularly strongly in hematopoietic organs. The major cells exhibiting HDC-induction were mast cells and c-kit+ cells in the bone marrow. HDC was also induced in non-mast cells in non-hematopoietic organs. HDC, histamine, and histamine H4 receptors (H4Rs) contributed to the suppression of IL-33-induced eosinophilia.
Conclusion
IL-33 directly and indirectly (via IL-5) induces HDC in various cells, particularly potently in c-kit+ cells and mature mast cells, and the newly formed histamine contributes to the negative regulation of IL-33-induced eosinophilia via H4Rs.







Similar content being viewed by others
Data availability
Research data are not shared.
References
Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–89.
Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–90.
Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–5.
Arend WP, Palmer G, Gabay C. IL-1, IL-18, and IL-33 families of cytokines. Immunol Rev. 2008;223:20–38.
Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–10.
Galand C, Leyva-Castillo JM, Yoon J, Han A, Lee MS, McKenzie ANJ, Stassen M, Oyoshi MK, Finkelman FD, Geha RS. IL-33 promotes food anaphylaxis in epicutaneously sensitized mice by targeting mast cells. J Allergy Clin Immunol. 2016;138:1356–66.
Nascimento DC, Melo PH, Piñeros AR, Ferreira RG, Colón DF, Donate PB, Castanheira FV, Gozzi A, Czaikoski PG, Niedbala W, Borges MC, Zamboni DS, Liew FY, Cunha FQ, Alves-Filho JC. IL-33 contributes to sepsis-induced long-term immunosuppression by expanding the regulatory T cell population. Nat Commun. 2017;8:14919.
Williams MA, O’Callaghan A, Corr SC. IL-33 and IL-18 in inflammatory bowel disease etiology and microbial interactions. Front Immunol. 2019;10:1091.
Taniguchi S, Elhance A, Van Duzer A, Kumar S, Leitenberger JJ, Oshimori N. Tumor-initiating cells establish an IL-33-TGF-beta niche signaling loop to promote cancer progression. Science. 2020;369:eaay1813.
Chen YL, Gutowska-Owsiak D, Hardman CS, Westmoreland M, MacKenzie T, Cifuentes L, Waithe D, Lloyd-Lavery A, Marquette A, Londei M, Ogg G. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci Transl Med. 2019;11:eaax2945.
Chinthrajah S, Cao S, Liu C, Lyu SC, Sindher SB, Long A, Sampath V, Petroni D, Londei M, Nadeau KC. Phase 2a randomized, placebo-controlled study of anti-IL-33 in peanut allergy. JCI Insight. 2019;4:e131347.
Kelsen SG, Agache IO, Soong W, Israel E, Chupp GL, Cheung DS, Theess W, Yang X, Staton TL, Choy DF, Fong A, Dash A, Dolton M, Pappu R, Brightling CE. Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol. 2021;148:790–8.
Kinbara M, Bando K, Shiraishi D, Kuroishi T, Nagai Y, Ohtsu H, Takano-Yamamoto T, Sugawara S, Endo Y. Mast cell histamine-mediated transient inflammation following exposure to nickel promotes nickel allergy in mice. Exp Dermatol. 2016;25:466–71.
Steelant B, Seys SF, Van Gerven L, Van Woensel M, Farré R, Wawrzyniak P, Kortekaas Krohn I, Bullens DM, Talavera K, Raap U, Boon L, Akdis CA, Boeckxstaens G, Ceuppens JL, Hellings PW. Histamine and T helper cytokine-driven epithelial barrier dysfunction in allergic rhinitis. J Allergy Clin Immunol. 2018;141:951-963.e8.
Bando K, Kuroishi T, Sugawara S, Endo Y. Interleukin-1 and histamine are essential for inducing nickel allergy in mice. Clin Exp Allergy. 2019;49:1362–73.
Schaper-Gerhardt K, Rossbach K, Nikolouli E, Werfel T, Gutzmer R, Mommert S. The role of the histamine H(4) receptor in atopic dermatitis and psoriasis. Br J Pharmacol. 2020;177:490–502.
Yamaga S, Yanase Y, Ishii K, Ohshimo S, Shime N, Hide M. Decreased intracellular histamine concentration and basophil activation in anaphylaxis. Allergol Int. 2020;69:78–83.
Yang XD, Ai W, Asfaha S, Bhagat G, Friedman RA, Jin G, Park H, Shykind B, Diacovo TG, Falus A, Wang TC. Histamine deficiency promotes inflammation-associated carcinogenesis through reduced myeloid maturation and accumulation of CD11b+Ly6G+ immature myeloid cells. Nat Med. 2011;17:87–95.
Endo Y, Kikuchi T, Takeda Y, Nitta Y, Rikiishi H, Kumagai K. GM-CSF and G-CSF stimulate the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunol Lett. 1992;33:9–13.
Endo Y, Nakamura M, Nitta Y, Kumagai K. Effects of macrophage depletion on the induction of histidine decarboxylase by lipopolysaccharide, interleukin 1 and tumour necrosis factor. Br J Pharmacol. 1995;114:187–93.
Yamaguchi K, Motegi K, Kurimoto M, Endo Y. Induction of the activity of the histamine forming enzyme, histidine decarboxylase, in mice by IL-18 and by IL-18 plus IL-12. Inflamm Res. 2000;49:513–9.
Kikuchi H, Watanabe M, Endo Y. Induction by interleukin-1 (IL-1) of the mRNA of histidine decarboxylase, the histamine-forming enzyme, in the lung of mice in vivo and the effect of actinomycin D. Biochem Pharmacol. 1997;53:1383–8.
Lebel B, Schneider E, Piquet-Pelllorce C, Machavoine F, Kindler V, Luffau G, Dy M. Antigenic challenge of immunized mice induces endogenous production of IL-3 that increases histamine synthesis in hematopoietic organs. J Immunol. 1990;145:1222–6.
Dy M, Schneider E. Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rev. 2004;25:393–410.
Schayer RW. Induced synthesis of histamine, microcirculatory regulation, and the mechanism of action of the adrenal glucocorticoid hormones. Progr Allergy. 1963;7:187–212.
Kahlson G, Rosengren E. New approaches to the physiology of histamine. Physiol Rev. 1968;48:155–96.
Endo Y. Simultaneous induction of histidine and ornithine decarboxylases and changes in their product amines following the injection of Escherichia coli lipopolysaccharide into mice. Biochem Pharmacol. 1982;31:1643–7.
Endo Y, Suzuki R, Kumagai K. Macrophages can produce factors capable of inducing histidine decarboxylase, a histamine-forming enzyme, in vivo in the liver, spleen and lung of mice. Cell Immunol. 1986;97:13–22.
Huang H, Li Y, Liang J, Finkelman FD. Molecular regulation of histamine synthesis. Front Immunol. 2018;20:1392.
Hirasawa N. Expression of histidine decarboxylase and its roles in inflammation. Int J Mol Sci. 2019;20:376.
Schirmer B, Neumann D. The function of the histamine H4 receptor in inflammatory and inflammation-associated diseases of the gut. Int J Mol Sci. 2021;22:6116.
Pushparaj PN, Tay HK, H’ng SC, Pitman N, Xu D, McKenzie A, Liew FY, Melendez AJ. The cytokine interleukin-33 mediates anaphylactic shock. Proc Natl Acad Sci. 2009;106:9773–8.
Komai-Koma M, Brombacher F, Pushparaj PN, Arendse B, McSharry C, Alexander J, Chaudhuri R, Thomson NC, McKenzie ANJ, McInnes I, Liew FY, Xu D. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via Interleukin-4 in na ̈ıve mice. Allergy. 2012;67:1118–26.
Haenuki Y, Matsushita K, Futatsugi-Yumikura S, Ishii KJ, Kawagoe T, Imoto Y, Fujieda S, Yasuda M, Hisa Y, Akira S, Nakanishi K, Yoshimoto T. A critical role of IL-33 in experimental allergic rhinitis. J Allergy Clin Immunol. 2012;130:184-94.e11.
Schneider E, Petit-Bertron A-F, Bricard R, Levasseur M, Ramadan A, Girard J-P, Herbelin A, Dy M. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J Immunol. 2009;183:3591–7.
Babina M, Wang Z, Franke K, Guhl S, Artuc M, Zuberbier T. Yin-Yang of IL-33 in human skin mast cells: reduced degranulation, but augmented histamine synthesis through p38 activation. J Invest Dermatol. 2019;139:1516-1525.e3.
Ohtsu H, Tanaka S, Terui T, Hori Y, Makabe-Kobayashi Y, Pejler G, Tchougounova E, Hellman L, Gertsenstein M, Hirasawa N, Sakurai E, Buzás E, Kovács P, Csaba G, Kittel A, Okada M, Hara ML, Numayama-Tsuruta K, Ishigaki-Suzuki S, Ohuchi K, Ichikawa A, Falus A, Watanabe T, Nagy A. Mice lacking histidine decarboxylase exhibit abnormal mast cells. FEBS Lett. 2001;502:53–6.
Horai R, Asano M, Sudo K, Kanuka H, Suzuki M, Nishihara M, Takahashi M, Iwakura Y. Production of mice deficient in genes for interleukin (IL)-1α, IL-1β, IL-1α/β, and IL-1 receptor antagonist shows that IL-1β is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187:1463–75.
Takai J, Ohtsu H, Sato A, Uemura S, Fujimura T, Yamamoto M, Moriguchi T. Lipopolysaccharide-induced expansion of histidine decarboxylase-expressing Ly6G+ myeloid cells identified by exploiting histidine decarboxylase BAC-GFP transgenic mice. Sci Rep. 2019;9:15603.
Endo Y. A simple method for the determination of polyamines and histamine and its application to the assay of ornithine decarboxylase and histidine decarboxylase activities. Method Enzymol. 1983;94:42–7.
Endo Y, Kikuchi T, Nakamura M, Shinoda H. Determination of histamine and polyamines in calcified tissues of mice: contribution of mast cells and histidine decarboxylase to the amount of histamine in the bone. Calcif Tissue Int. 1992;51:67–71.
Ito T, Smrz D, Jung MY, Bandara G, Desai A, Smrzova S, Kuehn HS, Beaven MA, Metcalfe DD, Gilfillan AM. Stem cell factor programs the mast cell activation phenotype. J Immunol. 2012;188:5428–37.
Bando K, Tanaka Y, Kuroishi T, Sasaki K, Yamamoto TT, Sugawara S, Endo Y. Mouse model of hydroquinone hypersensitivity via innate and acquired immunity and its promotion by combined reagents. J Invest Dermatol. 2017;137:1082–93.
Willebrand R, Voehringer D. IL-33-Induced cytokine secretion and survival of mouse eosinophils is promoted by autocrine GM-CSF. PLoS ONE. 2016;11:e0163751.
Salachas F, Schneider E, Lemoine FM, Lebel B, Daëron M, Navarro S, Ziltener H, Dy M. Aggregated IgE mimic interleukin-3-induced histamine synthesis by murine hematopoietic progenitors. Blood. 1994;4:1098–107.
Grootens J, Ungerstedt JS, Nilsson G, Dahlin JS. Deciphering the differentiation trajectory from hematopoietic stem cells to mast cells. Blood Adv. 2018;2:2273–81.
Cheng H, Zheng Z, Cheng T. New paradigms on hematopoietic stem cell differentiation. Protein Cell. 2020;11:34–44.
Endo Y, Nakamura M. The effects of LPS, IL-1 and TNFα on the hepatic accumulation of 5HT and platelets in the mouse. Br J Pharmacol. 1992;105:613–9.
Endo Y, Tabata T, Kuroda H, Tadano T, Matsushima K, Watanabe M. Induction of histidine decarboxylase in skeletal muscle in mice by electrical stimulation, prolonged walking and interleukin-1. J Physiol. 1998;509:587–98.
Johnston LK, Hsu CL, Krier-Burris RA, Chhiba KD, Chien KB, McKenzie A, Berdnikovs S, Bryce PJ. IL-33 precedes IL-5 in regulating eosinophil commitment and is required for eosinophil homeostasis. J Immunol. 2016;197:3445–53.
Yang ZP, Ling DY, Xie YH, Wu WX, Li JR, Jiang J, Zheng JL, Fan YH, Zhang Y. The association of serum IL-33 and sST2 with breast cancer. Dis Markers. 2015;2015:516895.
Terras S, Opitz E, Moritz RK, Höxtermann S, Gambichler T, Kreuter A. Increased serum IL-33 levels may indicate vascular involvement in systemic sclerosis. Ann Rheum Dis. 2013;72:144–5.
Mitsui A, Tada Y, Takahashi T, Shibata S, Kamata M, Miyagaki T, Fujita H, Sugaya M, Kadono T, Sato S, Asano Y. Serum IL-33 levels are increased in patients with psoriasis. Clin Exp Dermatol. 2016;41:183–9.
Akcay A, Nguyen Q, He Z, Turkmen K, Won Lee D, Hernando AA, Altmann C, Toker A, Pacic A, Ljubanovic DG, Jani A, Faubel S, Edelstein CL. IL-33 exacerbates acute kidney injury. J Am Soc Nephrol. 2011;22:2057–67.
Komai-Koma M, Gilchrist DS, McKenzie AN, Goodyear CS, Xu D, Liew FY. IL-33 activates B1 cells and exacerbates contact sensitivity. J Immunol. 2011;186:2584–91.
Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–8.
Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY. IL-33 exacerbates eosinophil- mediated airway inflammation. J Immunol. 2010;185:3472–80.
Dominguez D, Ye C, Geng Z, Chen S, Fan J, Qin L, Long A, Wang L, Zhang Z, Zhang Y, Fang D, Kuzel TM, Zhang B. Exogenous IL-33 restores dendritic cell activation and maturation in established cancer. J Immunol. 2017;198:1365–75.
Alves-Filho JC, Sônego F, Souto FO, Freitas A, Verri WA Jr, Auxiliadora-Martins M, Basile- Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708–12.
Fu AK, Hung KW, Yuen MY, Zhou X, Mak DS, Chan IC, Cheung TH, Zhang B, Fu WY, Liew FY, Ip NY. IL-33 ameliorates Alzheimer’s disease-like pathology and cognitive decline. Proc Natl Acad Sci USA. 2016;113:E2705–13.
Wu X, Yoshida A, Sasano T, Iwakura Y, Endo Y. Histamine production via mast cell-independent induction of histidine decarboxylase in response to lipopolysaccharide and interleukin-1. Int Immunopharmacol. 2004;4:513–20.
Le H, Kim W, Kim J, Cho HR, Kwon B. Interleukin-33: a mediator of inflammation targeting hematopoietic stem and progenitor cells and their progenies. Front Immunol. 2013;4:104.
Capitano ML, Griesenauer B, Guo B, Cooper S, Paczesny S, Broxmeyer HE. The IL-33 receptor/ST2 acts as a positive regulator of functional mouse bone marrow hematopoietic stem and progenitor cells. Blood Cells Mol Dis. 2020;84:102435.
Swann JW, Koneva LA, Regan-Komito D, Sansom SN, Powrie F, Griseri T. IL-33 promotes anemia during chronic inflammation by inhibiting differentiation of erythroid progenitors. J Exp Med. 2020;217:e20200164.
Chen X, Deng H, Churchill MJ, Luchsinger LL, Du X, Chu TH, Friedman RA, Middelhoff M, Ding H, Tailor YH, Wang ALE, Liu H, Niu Z, Wang H, Jiang Z, Renders S, Ho SH, Shah SV, Tishchenko P, Chang W, Swayne TC, Munteanu L, Califano A, Takahashi R, Nagar KK, Renz BW, Worthley DL, Westphalen CB, Hayakawa Y, Asfaha S, Borot F, Lin CS, Snoeck HW, Mukherjee S, Wang TC. Bone marrow myeloid cells regulate myeloid-biased hematopoietic stem cells via a histamine-dependent feedback loop. Cell Stem Cell. 2017;21:747-760.e7.
Dahlin JS, Hamey FK, Pijuan-Sala B, Shepherd M, Lau WWY, Nestorowa S, Weinreb C, Wolock S, Hannah R, Diamanti E, Kent DG, Göttgens B, Wilson NK. A single-cell hematopoietic landscape resolves 8 lineage trajectories and defects in Kit mutant mice. Blood. 2018;131:e1–11.
Inami Y, Sasaki A, Andoh T, Kuraishi Y. Surfactant-induced chronic pruritus: role of L-histidine decarboxylase expression and histamine production in epidermis. Acta Derm Venereol. 2014;94:645–50.
Gutowska-Owsiak D, Greenwald L, Watson C, Selvakumar TA, Wang X, Ogg GS. The histamine synthesizing enzyme histidine decarboxylase is upregulated by keratinocytes in atopic skin. Br J Dermatol. 2014;171:771–8.
Deng X, Wu X, Yu Z, Arai I, Sasano T, Sugawara S, Endo Y. Inductions of histidine decarboxylase in mouse tissues following systemic antigen-challenge: contributions made by mast cells, non-mast cells, and IL-1. Int Arch Allergy Immunol. 2007;144:69–78.
Koarai A, Ichinose M, Ishigaki-Suzuki S, Yamagata S, Sugiura H, Sakurai E, Makabe-Kobayashi Y, Kuramasu A, Watanabe T, Shirato K, Hattori T, Ohtsu H. Disruption of L-histidine decarboxylase reduces airway eosinophilia but not hyperresponsiveness. Am J Respir Crit Care Med. 2003;167:758–63.
Kozma GT, Losonczy G, Keszei M, Komlósi Z, Buzás E, Pállinger E, Appel J, Szabó T, Magyar P, Falus A, Szalai C. Histamine deficiency in gene-targeted mice strongly reduces antigen-induced airway hyper-responsiveness, eosinophilia and allergen-specific IgE. Int Immunol. 2003;15:963–73.
Ling P, Ngo K, Nguyen S, Thurmond RL, Edwards JP, Karlsson L, Fung-Leung WP. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br J Pharmacol. 2004;142:161–71.
Swartzendruber JA, Byrne AJ, Bryce PJ. Cutting edge: histamine is required for IL-4-driven eosinophilic allergic responses. J Immunol. 2012;188:536–40.
Hasala H, Giembycz MA, Janka-Junttila M, Moilanen E, Kankaanranta H. Histamine reverses IL-5-afforded human eosinophil survival by inducing apoptosis: pharmacological evidence for a novel mechanism of action of histamine. Pulm Pharmacol Ther. 2008;21:222–33.
Byron JW. Mechanism for histamine H2-receptor induced cell-cycle changes in the bone marrow stem cell. Agents Actions. 1977;7:209–13.
Nakaya N, Tasaka K. The influence of histamine on precursors of granulocytic leukocytes in murine bone marrow. Life Sci. 1988;42:999–1010.
Schneider E, Piquet-Pellorce C, Dy M. New role for histamine in interleukin-3-induced proliferation of hematopoietic stem cells. J Cell Physiol. 1990;143:337–43.
Schneider E, Ploemacher RE, Nabarra B, Brons NH, Dy M. Mast cells and their committed precursors are not required for interleukin-3-induced histamine synthesis in murine bone marrow: characteristics of histamine-producing cells. Blood. 1993;81:1161–9.
Petit-Bertron A-F, Machavoine F, Defresne MP, Gillard M, Chatelain P, Mistry P, Schneider E, Dy M. H4 histamine receptors mediate cell cycle arrest in growth factor-induced murine and human hematopoietic progenitor cells. PLoS ONE. 2009;4:e6054.
Schneider E, Bertron A-F, Dy M. Modulation of hematopoiesis through histamine receptor signaling. Front Biosci. 2011;S3:467–73.
Liu C, Ma X-J, Jiang X, Wilson SJ, Hofstra CL, Blevitt J, Pyati J, Li X, Chai W, Carruthers N, Lovenberg TW. Cloning and pharmacological characterization of a fourth histamine receptor (H4) expressed in bone marrow. Mol Pharmacol. 2001;59:420–6.
Bando K, Tanaka Y, Mizoguchi I, Sugawara S, Endo Y. Histamine acts via H4-receptor stimulation to cause augmented inflammation when lipopolysaccharide is co-administered with a nitrogen-containing bisphosphonate. Inflamm Res. 2022;71:1603–17.
Kenswil KJG, Jaramillo AC, Ping Z, Chen S, Hoogenboezem RM, Mylona MA, Adisty MN, Bindels EMJ, Bos PK, Stoop H, Lam KH, van Eerden B, Cupedo T, Raaijmakers MHGP. Characterization of endothelial cells associated with hematopoietic niche formation in humans identifies IL-33 as an anabolic factor. Cell Rep. 2018;22:666–78.
Acknowledgements
We are grateful to Dr. Robert Timms for editing the manuscript. We thank the Biomedical Research Core of Tohoku University Graduate School of Medicine for the use of its equipment. We thank Dr. Takashi Moriguchi and Dr. Jun Takai for providing HDC-GFP mice.
Funding
This work was supported by Grants from the Japan Society for the Promotion of Science [18K17240 and 21K10157 (Bando Kanan)].
Author information
Authors and Affiliations
Contributions
Study was designed and conducted by KB, YT, SS, IM, and YE. Data collected by KB. Data analyzed by KB and YE. Technically assisted by YT and SW. Manuscript written by KB and YE and approved by all authors.
Corresponding author
Ethics declarations
Conflict of interest
Authors have no conflicts of interest in this study.
Ethics approval
All animal procedures were approved by the Institutional Animal Care and Use Committee of Tohoku University (approval number: 2018DnA-013). All experiments complied with the Regulations for Animal Experiments and Related Activities at Tohoku University.
Consent for publication
Not applicable.
Additional information
Responsible Editor: Bernhard Gibbs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bando, K., Tanaka, Y., Winias, S. et al. IL-33 induces histidine decarboxylase, especially in c-kit+ cells and mast cells, and roles of histamine include negative regulation of IL-33-induced eosinophilia. Inflamm. Res. 72, 651–667 (2023). https://doi.org/10.1007/s00011-023-01699-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01699-y