Abstract
Background
Mast cells utilize SNAREs (soluble-N-ethyl-maleimide sensitive factor attachment protein receptors) and SM (Sec1/Munc18) proteins to secrete/exocytose a variety of proinflammatory mediators. However, whether a common SNARE-SM machinery is responsible remains unclear.
Methods
Four vesicle/granule-anchored SNAREs (VAMP2, VAMP3, VAMP7, and VAMP8) and two Munc18 homologs (Munc18a and Munc18b) were systematically knocked down or knocked out in RBL-2H3 mast cells and antigen-induced release of β-hexosaminidase, histamine, serotonin, and TNF was examined. Phenotypes were validated by rescue experiments. Immunofluorescence studies were performed to determine the subcellular distribution of key players.
Results
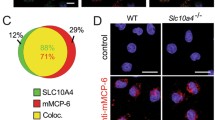
The reduction of VAMP8 expression inhibited the exocytosis of β-hexosaminidase, histamine, and serotonin but not TNF. Unexpectedly, however, confocal microscopy revealed substantial co-localization between VAMP8 and TNF, and between TNF and serotonin. Meanwhile, the depletion of other VAMPs, including knockout of VAMP3, had no impact on the release of any of the mediators examined. On the other hand, TNF exocytosis was diminished specifically in stable Munc18bknockdown cells, in a fashion that was rescued by exogenous, RNAi-resistant Munc18b. In line with this, TNF was co-localized with Munc18b (47%) to a much greater extent than with Munc18a (13%).
Conclusion
Distinct exocytic pathways exist in mast cells for the release of different mediators.







Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Ann N Y Acad Sci. 2008;1143:83–104.
Gilfillan AM, Beaven MA. Regulation of mast cell responses in health and disease. Crit Rev Immunol. 2011;31(6):475–529.
Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–94.
Lundequist A, Pejler G. Biological implications of preformed mast cell mediators. Cell Mol Life Sci. 2011;68(6):965–75.
Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. 2014;5:569.
Jahn R, Scheller RH. SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–43.
Rothman JE. The principle of membrane fusion in the cell (Nobel lecture). Angew Chem Int Ed Engl. 2014;53(47):12676–94.
Han J, Pluhackova K, Bockmann RA. The Multifaceted Role of SNARE Proteins in Membrane Fusion. Front Physiol. 2017;8:5.
Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323(5913):474–7.
Rizo J, Sudhof TC. The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices–guilty as charged? Annu Rev Cell Dev Biol. 2012;28:279–308.
Xu H, et al. HOPS prevents the disassembly of trans-SNARE complexes by Sec17p/Sec18p during membrane fusion. EMBO J. 2010;29(12):1948–60.
Baker RW, et al. A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly. Science. 2015;349(6252):1111–4.
Rathore SS, et al. Topological arrangement of the intracellular membrane fusion machinery. Mol Biol Cell. 2011;22(14):2612–9.
Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490(7419):201–7.
Woska JR, Gillespie ME. Small-interfering RNA-mediated identification and regulation of the ternary SNARE complex mediating RBL-2H3 mast cell degranulation. Scand J Immunol. 2011;73(1):8–17.
Paumet F, et al. Soluble NSF attachment protein receptors (SNAREs) in RBL-2H3 mast cells: functional role of syntaxin 4 in exocytosis and identification of a vesicle-associated membrane protein 8-containing secretory compartment. J Immunol. 2000;164(11):5850–7.
Tiwari N, et al. VAMP-8 segregates mast cell-preformed mediator exocytosis from cytokine trafficking pathways. Blood. 2008;111(7):3665–74.
Tiwari N, et al. Increased formation of VAMP-3-containing SNARE complexes in mast cells from VAMP-8 deficient cells. Inflamm Res. 2009;58(Suppl 1):13–4.
Brochetta C, et al. Munc18-2 and syntaxin 3 control distinct essential steps in mast cell degranulation. J Immunol. 2014;192(1):41–51.
Sander LE, et al. Vesicle associated membrane protein (VAMP)-7 and VAMP-8, but not VAMP-2 or VAMP-3, are required for activation-induced degranulation of mature human mast cells. Eur J Immunol. 2008;38(3):855–63.
Suzuki K, Verma IM. Phosphorylation of SNAP-23 by IkappaB kinase 2 regulates mast cell degranulation. Cell. 2008;134(3):485–95.
Hibi T, Hirashima N, Nakanishi M. Rat basophilic leukemia cells express syntaxin-3 and VAMP-7 in granule membranes. Biochem Biophys Res Commun. 2000;271(1):36–41.
Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94(4):537–48.
Tadokoro S, et al. Munc18-2 regulates exocytotic membrane fusion positively interacting with syntaxin-3 in RBL-2H3 cells. Mol Immunol. 2007;44(13):3427–33.
Martin-Verdeaux S, et al. Evidence of a role for Munc18-2 and microtubules in mast cell granule exocytosis. J Cell Sci. 2003;116(Pt 2):325–34.
Bin NR, et al. Crucial role of the hydrophobic pocket region of Munc18 protein in mast cell degranulation. Proc Natl Acad Sci USA. 2013;110(12):4610–5.
Nigam R, et al. Expression and transcriptional regulation of Munc18 isoforms in mast cells. Biochim Biophys Acta. 2005;1728(1–2):77–83.
Xu H, Arnold MG, Kumar SV. Differential effects of Munc18s on multiple degranulation-relevant trans-SNARE complexes. PLoS ONE. 2015;10(9): e0138683.
Dvorak AM. Human mast cells. Adv Anat Embryol Cell Biol. 1989;114:1–107.
Raposo G, et al. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Mol Biol Cell. 1997;8(12):2631–45.
Theoharides TC, et al. Differential release of serotonin and histamine from mast cells. Nature. 1982;297(5863):229–31.
Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346(6281):274–6.
Gordon JR, Galli SJ. Release of both preformed and newly synthesized tumor necrosis factor alpha (TNF-alpha)/cachectin by mouse mast cells stimulated via the Fc epsilon RI. A mechanism for the sustained action of mast cell-derived TNF-alpha during IgE-dependent biological responses. J Exp Med. 1991;174(1):103–7.
Metcalfe DD, Peavy RD, Gilfillan AM. Mechanisms of mast cell signaling in anaphylaxis. J Allergy Clin Immunol. 2009;124(4):639–46.
Rivera, J. and A.M. Gilfillan, Molecular regulation of mast cell activation. J Allergy Clin Immunol, 2006. 117(6): p. 1214–25; quiz 1226.
Bin NR, et al. C2 domains of Munc13-4 are crucial for Ca(2+)-dependent degranulation and cytotoxicity in NK cells. J Immunol. 2018;201(2):700–13.
Horton RM, et al. Gene splicing by overlap extension. Methods Enzymol. 1993;217:270–9.
Malmersjo S, et al. Phosphorylation of residues inside the SNARE complex suppresses secretory vesicle fusion. EMBO J. 2016;35(16):1810–21.
Ayo TE, et al. TNF production in activated RBL-2H3 cells requires Munc13-4. Inflammation. 2020;43(2):744–51.
Higashio H, Satoh YI, Saino T. Inhibitory role of Munc13-1 in antigen-induced mast cell degranulation. Biomed Res. 2017;38(6):321–9.
Blank U, et al. Vesicular trafficking and signaling for cytokine and chemokine secretion in mast cells. Front Immunol. 2014;5:453.
Murray RZ, et al. A role for the phagosome in cytokine secretion. Science. 2005;310(5753):1492–5.
Boddul SV, et al. SNAP-23 and VAMP-3 contribute to the release of IL-6 and TNFalpha from a human synovial sarcoma cell line. FEBS J. 2014;281(3):750–65.
Caceres PS, et al. Vesicle-associated membrane protein 3 (VAMP3) mediates constitutive trafficking of the renal co-transporter NKCC2 in thick ascending limbs: role in renal function and blood pressure. J Biol Chem. 2016;291(42):22063–73.
Sanchez E, et al. Syntaxin 3, but not syntaxin 4, is required for mast cell-regulated exocytosis, where it plays a primary role mediating compound exocytosis. J Biol Chem. 2019;294(9):3012–23.
Puri N, Roche PA. Mast cells possess distinct secretory granule subsets whose exocytosis is regulated by different SNARE isoforms. Proc Natl Acad Sci USA. 2008;105(7):2580–5.
Gutierrez BA, et al. Munc18-2, but not Munc18-1 or Munc18-3, controls compound and single-vesicle-regulated exocytosis in mast cells. J Biol Chem. 2018;293(19):7148–59.
Lorentz A, et al. The SNARE machinery in mast cell secretion. Front Immunol. 2012;3:143.
Wade N, et al. Syntaxin 7 complexes with mouse Vps10p tail interactor 1b, syntaxin 6, vesicle-associated membrane protein (VAMP)8, and VAMP7 in b16 melanoma cells. J Biol Chem. 2001;276(23):19820–7.
Jean S, et al. Starvation-induced MTMR13 and RAB21 activity regulates VAMP8 to promote autophagosome-lysosome fusion. EMBO Rep. 2015;16(3):297–311.
Pryor PR, et al. Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Rep. 2004;5(6):590–5.
Mishima S, et al. Multifunctional regulation of VAMP3 in exocytic and endocytic pathways of RBL-2H3 cells. Front Immunol. 2022;13: 885868.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases Grant R15AI133430-01 to H.X. and S.S, and by the Mississippi INBRE, which was funded by an Institutional Development Award from the National Institute of General Medical Sciences under grant no. P20GM103476. We thank Dr. Seth Malmersjö for kindly providing the RNAi-resistant VAMP8 rescue construct and Dr. Jonathan Lindner at the INBRE Imaging Facility for excellent technical support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by PA, TA, and SS. Data collection and analysis were conducted by PA, TA, HX, and JV. The first draft of the manuscript was written by HX, PA, and TA. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Bernhard Gibbs.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Adhikari, P., Ayo, T.E., Vines, J.C. et al. Exocytic machineries differentially control mediator release from allergen-triggered RBL-2H3 cells. Inflamm. Res. 72, 639–649 (2023). https://doi.org/10.1007/s00011-023-01698-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-023-01698-z