Abstract
Background and Objective
The availability of different tyrosine kinase inhibitors (TKIs) with distinct antileukemic potency enables optimization of current therapeutic regimens; however, some patients lose their therapy response and acquire TKI resistance. In this study, we describe a single-center experience of monitoring BCR-ABL1 kinase domain (KD) mutations and discuss the impact of treatment on mutation selection.
Methods
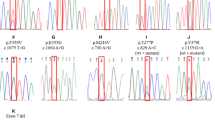
Chronic myelogenous leukemia (CML) patients treated with TKIs at the Department of Internal Medicine — Hematology and Oncology, Masaryk University and University Hospital Brno during 2003–2011 were included in this study. A total number of 100 patients who did not achieve an optimal therapy response or who lost their therapy response were screened for the presence of BCR-ABL1 KD mutations, using direct sequencing.
Results
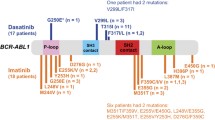
Our data show that pretreatment with non-specific non-TKI drugs prior to TKI therapy does not preferentially select for initial BCR-ABL1 KD mutations, in contrast to first-line imatinib therapy, which shows a clear predominance of T315I or P-loop mutations compared with mutations located in other KD regions. In addition, the median time to detection of P-loop mutations was substantially shorter in patients treated with first-line imatinib than in those pretreated with non-TKI drugs. Furthermore, analysis of CML patients who had recurrent resistance to TKI therapy revealed possible therapy-driven selection of BCR-ABL1 KD mutations. Finally, we confirm the previously described poor prognosis of CML patients with mutations in the BCR-ABL1 KD, since 40.0% of our CML patients who harbored a BCR-ABL1 KD mutation died from CML while receiving TKI treatment. Moreover, among the patients who are still on treatment, 27.8% have already progressed. Our data also confirm the unique position of the T315I mutation with respect to its strong resistance to currently approved TKIs.
Conclusion
On the basis of the ‘real-life’ data described in this study, it is possible that the therapy itself results in its failure and selects the most resistant mutations under the selective pressure of the applied therapy regimen in some CML patients who harbor BCR-ABL1 KD mutations.







Similar content being viewed by others
References
Deininger M, O’Brien SG, Guilhot F, et al. International Randomized Study of Interferon Vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [abstract no. 1126]. Blood (ASH Annual Meeting Abstracts) 2009; 114: Abstract 1126.
Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood 2007; 109: 2303–9.
Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood 2007; 110: 3540–6.
Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol 2007; 8: 1018–29.
Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood 2009; 114: 4944–53.
Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol 2009; 27: 4204–10.
Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 2003; 102: 276–83.
Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001; 293: 876–80.
Branford S, Rudzki Z, Walsh S, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood 2002; 99: 3472–5.
Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell 2002; 2: 117–25.
Branford S. Chronic myeloid leukemia: molecular monitoring in clinical practice. Hematology Am Soc Hematol Educ Program 2007; 376–83.
Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res 2006; 12: 7374–9.
Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia 2006; 20: 1767–73.
Nicolini FE, Corm S, Le QH, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukemia patients: a retrospective analysis from the French Intergroup of CML (Fi(phi)-LMC GROUP). Leukemia 2006; 20: 1061–6.
Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood 2009; 114: 5426–35.
Valent P. Targeting of leukemia-initiating cells to develop curative drug therapies: straightforward but nontrivial concept. Curr Cancer Drug Targets 2011; 11: 56–71.
Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant Bcr-Abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood 2009; 114: 2168–71.
Shah NP, Skaggs BJ, Branford S, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. Clin Invest 2007; 117: 2562–9.
Cortes J, Jabbour E, Kantarjian H, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood 2007; 110: 4005–11.
Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009; 27: 6041–51.
Poláková KM, Polívková V, Rulcová J, et al. Constant BCR-ABL transcript level ≥0.1% (IS) in patients with CML responding to imatinib with complete cytogenetic remission may indicate mutation analysis. Exp Hematol 2010; 38: 20–6.
Gabert J, Beillard E, van derVelden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia — a Europe Against Cancer program. Leukemia 2003; 17: 2318–57.
Beillard E, Pallisgaard N, van derVelden VH, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) — a Europe Against Cancer program. Leukemia 2003; 17: 2474–86.
Poláková KM, Lopotová T, Klamová H, et al. High-resolution melt curve analysis: initial screening for mutations in BCR-ABL kinase domain. Leuk Res 2008; 32: 1236–43.
Nicolini FE, Corm S, Le Q-H, et al. The prognosis impact of BCR-ABL P-loop mutations: worse or not worse? Leukemia 2007; 21: 193–4.
Cang S, Liu D. P-loop mutations and novel theraputic approaches for imatinib failures in chronic myeloid leukemia. J Hematol Oncol 2008; 1:15.
Paget S. The distribution of secondary growths in cancer of the breast. Lancet 1889; 133 (3421): 571–3.
Lin EH, Jiang Y, Deng Y, et al. Cancer stem cells, endothelial progenitors, and mesenchymal stem cells: “seed and soil” theory revisited. Gastrointest Cancer Res 2008; 2: 169–74.
Essers MA, Ordner S, Blanco-Bose WE, et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 2009; 458: 904–8.
Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood 2002; 99: 319–25.
Michor F, Hughes TP, Iwasa Y, et al. Dynamics of chronic myeloid leukaemia. Nature 2005; 435: 1267–70.
Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood 2006; 107: 4532–9.
Jorgensen HG, Allan EK, Jordanides NE, et al. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood 2007; 109: 4016–9.
Kantarjian HM, O’Brien S, Cortes J, et al. Imatinib mesylate therapy improves survival in patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in the chronic phase: comparison with historic data. Cancer 2003; 98: 2636–42.
Kantarjian H, Talpaz M, O’Brien S, et al. Survival benefit with imatinib mesylate therapy in patients with accelerated-phase chronic myelogenous leukemia -comparison with historic experience. Cancer 2005; 103: 2099–108.
Khorashad JS, deLavallade H, Apperley JF, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol 2008; 26: 4806–13.
Soverini S, Iacobucci I, Baccarani M, et al. Targeted therapy and the T315I mutation in Philadelphia-positive leukemias. Haematologica 2007; 92: 437–9.
Azam M, Seeliger MA, Gray NS, et al. Activation of tyrosine kinases by mutation of the gatekeeper threonine. Nat Struct Mol Biol 2008; 15: 1109–18.
O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell 2009; 16: 401–12.
Gruber FX, Ernst T, Porkka K, et al., Dynamics of the emergence of dasatinib and nilotinib resistance in imatinib-resistant CML patients. Leukemia 2012; 26: 172–7.
Quintas-Cardama AG, Gibbons DL, Kantarjian H, et al. Mutational analysis of chronic myeloid leukemia (CML) clones reveals heightened BCRABL1 genetic instability and wild-type BCR-ABL1 exhaustion in patients failing sequential imatinib and dasatinib therapy [abstract no. 1938]. Blood (ASH Annual Meeting Abstracts) 2007; 110: 1938.
Naka K, Hoshii A, Hirao A. Novel therapeutic approach to eradicate tyrosine kinase inhibitor resistant chronic myeloid leukemia stem cells. Cancer Sci 2010; 101: 1577–81.
Azam M, Powers JT, Einhorn W, et al. AP24163 inhibits the gatekeeper mutant of BCR-ABL and suppresses in vitro resistance. Chem Biol Drug Des 2010; 75: 223–7.
Acknowledgments
Filip Razga and Tomas Jurcek equally contributed to this work. The work was supported by the CzEch Leukemia Study Group for Life (CELL), grant no. MSM0021622430 from the Ministry of Education, Youth, and Sports of the Czech Republic, and grant no. MUNI/A/0784/2011 from Masaryk University Brno. The authors have no conflicts of interest that are directly relevant to the content of this report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Razga, F., Jurcek, T., Zackova, D. et al. Role of Treatment in the Appearance and Selection of BCR-ABL1 Kinase Domain Mutations. Mol Diagn Ther 16, 251–259 (2012). https://doi.org/10.1007/BF03262214
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03262214