Abstract
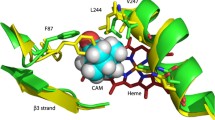
In structural studies of cytochrome P450 enzymes, substrates have been seen to bind in a variety of modes; it is important to identify those with the closest resemblance to the configurations adopted during selective oxidation. We attempt here to identify conditions in which the catalytic binding mode of cytochrome P450 BM-3 saturated with N-palmitoylglycine is highly populated. When the substrate binds directly atop the heme, primed for oxidation, displacement of the water ligand is necessary, and thereby the ferric heme is generally converted from low-spin to high-spin. Using both optical spectroscopy and solid-state nuclear magnetic resonance, studying both the full-length enzyme and the isolated heme domain, we show that a high population of the high-spin form is seen at room temperature and above, but not at reduced temperatures. In contrast, the reduced state exhibits high spin throughout the temperature range. The isotropic chemical shift of deuterons in the substrate bound to the oxidized and reduced forms of the enzyme was temperature-dependent, consistent with the presence of a nearby paramagnetic center, but temperature-independent for the diamagnetic CO-bound form, and for the free form of the compound. The reduced (ferrous heme) species shows Curie law dependence of the2H substrate chemical shift with respect to temperature from −54 to +35 °C, but the oxidized (ferric heme) species showed a pronounced non-Curie dependence in both the2H and the13C shift of the substrate’s methyl group, with the effect of the paramagnetic heme at low temperatures being much reduced. These data are consistent with a mixture of at least two binding modes in rapid equilibrium wherein the heme is high-spin at room temperature but low-spin at cryogenic temperatures.
Similar content being viewed by others
References
Guengerich F.P.: J. Biol. Chem.266, 10019–10022 (1991)
Wester M.R., Johnson E.F., Marques-Soares C., Dijols S., Dansette P.M., Mansuy D., Stout C.D.: Biochemistry42, 9335–9345 (2003)
Wester M.R., Johnson E.F., Marques-Soares C., Dansette P.M., Mansuy D., Stout C.D.: Biochemistry42, 6370–6379 (2003)
Williams P.A., Cosme J., Ward A., Angova H.C., Vinkovic D.M., Jhoti H.: Nature424, 464–468 (2003)
Wester M.R., Yano J.K., Schoch G.A., Yang C., Griffin K.J., Stout C.D., Johnson E.F.: J. Biol. Chem.279, 35630–35637 (2004)
Schoch G.A., Yano J.K., Wester M.R., Griffin K.J., Stout C.D., Johnson E.F.: J. Biol. Chem.279, 9497–9503 (2004)
Williams P.A., Cosme J., Vinkovic D.M., Ward A., Angove H.C., Day P.J., Vonrhein C., Tickle I.J., Jhoti H.: Science.305, 683–686 (2004)
Li H.Y., Poulos T.L.: Nat. Struct. Biol.4, 140–146 (1997)
Haines D.C., Tomchick D.R., Machius M., Peterson J.A.: Biochemistry40, 13456–13465 (2001)
Modi S., Sutcliffe M.J., Primrose W.U., Lian L.Y., Roberts G.C.K.: Nat. Struct. Biol.3, 414–417 (1996)
Modi S., Primrose W.U., Boyle J.M.B., Gibson C.F., Lian L.Y., Roberts G.C.K.: Biochemistry34, 8982–8988 (1995)
Estabrook R.W., Hildebrandt A.G., Baron J., Netter K.J., Leibman K.: Biochem. Biophys. Res. Commun.42, 132–139 (1971)
Daff S.N., Chapman S.K., Turner K.L., Holt R.A., Govindaraj S., Poulos T.L., Munro A.W.: Biochemistry36, 13816–13823 (1997)
Munro A.W., Daff S., Coggins J.R., Lindsay J.G., Chapman S.K.: Eur. J. Biochem.239, 403–409 (1996)
Sevrioukova I.F., Hazzard J.T., Tollin G., Poulos T.L.: J. Biol. Chem.274, 36097–36106 (1999)
Jovanovic T., McDermott A.E., Farid R., Friesner R.A.: J. Am. Chem. Soc.127, 13548–13552 (2005)
Hudeeek J., Baumruk V., Anzenbacher P., Munro A.W.: Biochem. Biophys. Res. Commun.243, 811–815 (1998)
Hudecek J., Anzenbacherova E., Anzenbacher P., Munro A.W., Hildebrandt P.: Arch. Biochem. Biophys.383, 70–78 (2000)
Smith P.K., Krohn R.I., Hermanson G.T., Mallia A.K., Gartner F.H., Provenzano M.D., Fujimoto E.K., Goeke N.M., Olson B.J., Klenk D.C.: Anal. Biochem.150, 76–85 (1985)
Omura T., Sato R.: J. Biol. Chem.239, 2370–2378 (1964)
Black S.D., Linger M.H., Freck L.C., Kazemi S., Galbraith J.A.: Arch. Biochem. Biophys.310, 126–133 (1994)
Schwaneberg U., Schmidt-Dannert C., Schmitt J., Schmid R.D.: Anal. Biochem.269, 359–366 (1999)
Lapidot Y., Rappopor S., Wolman Y.: J. Lipid Res.8, 142–145 (1967)
Zuo C.S., Metz K.R., Sun Y., Sherry A.D.: J. Magn. Reson.133, 53–60 (1998)
Denisov I.G., Makris T.M., Sligar S.G., Schlichting I.: Chem. Rev.105, 2253–2277 (2005)
Fang X.J., Halpert J.R.: Drug Metab Dispos.24, 1282–1285 (1996)
Peterson J.A., Boddupalli S.S.: Arch. Biochem. Biophys.294, 654–661 (1992)
Sober H.A.: Handbook of Biochemistry: Selected Data for Molecular Biology, p. 982. Cleveland, Ohio: Chem. Rubber Co. 1968.
Jovanovic T., McDermott A.E.: J. Am. Chem. Soc.127, 13816–13821 (2005)
Lee H., de Montellano P.R.O., McDermott A.E.: Biochemistry38, 10808–10813 (1999)
Jovanovic T., McDermott A.E. in: Proceedings of the 14th International Conference on Cytochromes P450: Biochemistry, Biophysics, and Bioinformatics, pp. 25–28, 14th International Conference on Cytochromes P450: Biochemistry, Biophysics, and Bioinformatics, Dallas, TX, USA 2005. Medimond S.r.l., Via Maserati 5, Bologna, Italy 2005.
McConnell H.M., Robertson R.E.: J. Chem. Phys.29, 1361–1365 (1958)
Beetlestone J., George P.: Biochemistry3, 707–714 (1964)
Iizuka T., Kotani M.: Biochim. Biophys. Acta154, 417–419 (1968)
Iizuka T., Kotani M.: Biochim. Biophys. Acta181, 275–286 (1969)
Griffith J.S.: Proc. R. Soc. Lond. A235, 23–36 (1956)
Iizuka T., Kotani M., Yonetani T.: Biochim. Biophys. Acta167, 257–267 (1968)
Joyce G.M., Girvan H.M., Munro A.W., Leys D.: J. Biol. Chem.279, 23287–23293 (2004)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jovanovic, T., Harris, M. & McDermott, A.E. Cytochrome P450 BM-3 in complex with its substrate: Temperature-dependent spin state equilibria in the oxidized and reduced states. Appl. Magn. Reson. 31, 411–429 (2007). https://doi.org/10.1007/BF03166593
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF03166593