Summary
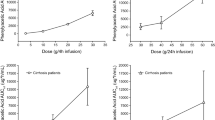
Renal toxicity was assessed in 19 patients receiving methyl acetylenic putrescine (MAP), an irreversible inhibitor of ornithine decarboxylase. Patients received 250 mg t. d. s. for up to 13 weeks. This dose effectively inhibited the target enzyme, as shown by elevations in decarboxylated S-adenosyl methionine levels. No significant nephrotoxicity was observed in these patients as determined by plasma urea, creatinine and creatinine clearance measurements, although minor elevations of the urinary enzymes lactate dehydrogenase,N-acetyl-β-glucosaminidase, alkaline phosphatase and alanine aminopeptidase were observed. As this could represent sub-clinical renal damage, caution should be excercised when using MAP in combination with other cytotoxic drugs.
Similar content being viewed by others
References
Bradford M (1970) A rapid and sensitive method for the quantitation of microgram quantities of protein. Anal Biochem 72: 248–254
Bretaudiere JP, Spillman J (1983) Alkaline phosphatases, routine methods in methods of enzymatic analysis, 3rd edn, vol IV. Verlag Chemie, Weinheim, pp 75–82
Carmichael J, Cantwell BMJ, Khayat D (1989) Phase 2 trial of methyl acetylenic putrescine in colorectal carcinoma: clinical and biochemical effects. Br J Cancer (in press)
Cornbleet MA, Kingsnorth A, Tell JP, Haegele KD, Joder-Ohlenbusch AM, Smyth JF (1990) Phase I study of methyl acetylenic putrescine; and inhibitor of polyamine synthesis. Cancer Chemother Pharmacol (in press)
Danzin C, Casara P, Claverie N, Metcalf BW, Jung MJ (1983) (2R,5R)-6 Heptyne-2,5-diamine, an extremely potent inhibitor of mammalian ornithine decarboxylase. Biochem Biophys Res Commun 166: 237–243
Diener U, Knoll E, Langer B (1981) Urinary excretion ofN-acetyl-β-d-glucosaminidase and alanine aminopeptidase in patients receiving amiacin or cisplatinum. Clin Chim Acta 112: 149–157
Henry RH (1964) Creatinine in clinical chemistry. Principles and techniques. Hoeber Medical Division, Harper and Row, New York, pp 292–299
Jones BR, Bhalla RB, Mladek J (1980) Comparison of methods of evaluating nephrotoxicity of cisplatinum. Clin Pharmacol Ther 27: 557–562
Jurg K, Scholz D (1980) An optimised assay of alanine aminopeptidase in urine. Clin Chem 26: 1251–1254
Maruhn D (1976) Rapid colorimetric assay ofB-galactosidase andN-acetyl glucosaminidase in human urine. Clin Chim Acta 73: 453–461
Pegg AE, McCann PP (1982) Polyamine metabolism and function. Am J Physiol 243: 212–221
Vassault M (1983) Lactate dehydrogenase: UV method with pyruvate and NADH in methods of enzymatic analysis. 3rd edn, vol II. Verlag Chemie, Weinheim, pp 118–126
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Carmichael, J., Cantwell, B.M.J., Harris, A.L. et al. Assessment of renal toxicity by urinary enzymes in patients receiving chemotherapy with 8-methyl-8-acetylenic-putrescine. Cancer Chemother Pharmacol 26, 65–66 (1990). https://doi.org/10.1007/BF02940297
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02940297