Abstract
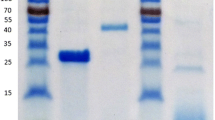
Thermomonospora fusca produced a relatively high level of α-l-arabinofuranosidase when growing on oat spelt xylan as the main carbon and energy source. The enzyme exhibited maximum relative activity (0.136 U/g protein) at pH 9.0 with 54 and 55% activity remaining at pH of 4.5 and 11.0, respectively. The apparentKm value for the crude α-l-arabinofuranosidase preparation was 180 µmol/L 4-nitrophenyl α-l-arabinofuranoside; thevlim value was the release of 40 µmol/L 4-nitrophenol per min. Enzyme activity was eluted as a single peak (HPLC gel filtration chromatography) corresponding to molar mass of ≈92 kDa. Native electrophoresis of crude cell lysate confirmed the presence of a single active intracellular α-l-arabinofuranosidase component. SDS-PAGE of this enzyme, developed as zymogram, did not demonstrate any activity; denaturing gel was stained and a protein band of relative molar mass of 46 kDa was revealed. Isoelectric focusing of a purified α-l-arabinofuranosidase yielded a single protein band for the corresponding activity zone with pI 7.9. The enzyme was purified approximately 21-fold the mean overall yield was about 16%.
Similar content being viewed by others
Abbreviations
- IEF:
-
isoelectric focusing
- NPA:
-
4-nitrophenyl α-l-arabinofuranoside
- PAGE:
-
polyacrylamide gel electrophoresis
- SDS:
-
sodium dodecyl sulfate
References
Bachmann S.L., McCarthy A.J.: Purification and characterization of a thermostable β-xylosidase fromThermomonospora fusca.J.Gen.Microbiol.135, 293–299 (1989).
Bachmann S.L., McCarthy A.J.: Purification and co-operative activity of enzymes constituting the xylan-degrading system ofThermomonospora fusca.Appl.Environ.Microbiol.57, 2121–2130 (1991).
Bèguin P.: Molecular biology of cellulose degradation.Ann.Rev.Microbiol.44, 219–248 (1990).
Bèguin P., Aubert J.P.: The biological degradation of cellulose.FEMS Microbiol.Rev.13, 25–58 (1994).
Betts W.B., Dart R.K., Ball A.S., Pedlar S.L.: Biosynthesis and structure of lignocellulose, pp. 139–156 in W.B. Betts (Ed.):Biodegradation Natural and Synthetic Materials. Springer-Verlag, London 1992.
Coughlan M.P., Hazlewood G.P.: β-1,4-d-Xylan-degrading enzyme systems: biochemistry, molecular biology and applications.Biotechnol.Appl.Biochem.17, 259–289 (1993).
Gilead S., Shoham Y.: Purification and characterization of α-l-arabinofuranosidase fromBacilius stearothermophilus T-6.Appl.Environ.Microbiol.61, 170–174 (1995).
Goodfellow M., Williams S.T.: Ecology of actinomycetes.Ann.Rev.Microbiol.37, 189–216 (1983).
Greve L.C., Labavitch J.M., Hungate R.E.: α-l-Arabinofuranosidase fromRuminococcus albus 8: purification and possible role in hydrolysis of alfalfa cell wall.Appl.Environ.Microbiol.47, 1135–1140 (1984).
Iqbal M., Mercer D.K., Miller P.G.G., McCarthy A.J.: Thermostable extra-cellular peroxidases fromStreptomyces thermoviolaceus.Microbiology140, 1457–1465 (1994).
Joseleau J.P., Comtat J., Ruel K.: Chemical structure of xylans and their interaction in the plant cell walls, pp. 1–15 in J. Visser, G. Beldman, M.A. Kusters van Somerenand, A.G.J. Voragen (Eds):Xylans and Xylanases. Progress in Biotechnology, Vol. 7. Elsevier Applied Science, Amsterdam (The Netherlands) 1992
Kaji A., Sato M., Tsutsui Y.: An α-l-arabinofuranosidase produced by the wild-typeStreptomyces sp. 17-1.Agr.Biol.Chem.45, 925–931 (1981).
Laemmli U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature227, 680–685 (1970).
Lee S.F., Forsberg C.W.: Purification and characterization of an α-l-arabinofuranosidase fromClostridium acetobutylicum ATCC 824.Can.J.Microbiol.33, 1011–1016 (1987).
Matsuo N., Kaneko S., Kuno A., Kobayashi H., Kusakabe I.: Purification, characterization end gene cloning of two α-l-arabinofuranosidase fromStreptomyces chartreusis GS901.Biochem.J.346, 9–15 (2000).
Morales P., Madarro A., Flors A., Sendra J.M., Pérez-González J.A.: Purification and characterization of a xylanase and an arabinofuranosidase fromBacillus polymyxa.Enzyme Microbiol.Technol.17, 424–429 (1995).
Nissen A.M., Anker L., Munk N., Lange N.K.: Xylanases for the pulp and paper industry, pp. 325–337 in J. Visser, G. Beldman, M.A. Kusters van Somerenand, A.G.J. Voragen (Eds):Xylans and Xylanases. Progress in Biotechnology, Vol. 7. Elsevier Applied Science, Amsterdam (The Netherlands) 1992.
Puls J., Poutanen K.: Mechanism of enzymatic hydrolysis of hemicelluloses (xylans) and procedures for determination of the enzyme activities involved, pp. 151–165 in M.P. Coughlan (Ed.),Enzyme Systems for Lignocellulose Degradation. Elsevier Applied Science, London 1989.
Ramachandra M., Crawford D.L., Pometto A.L.: Extracellular enzyme activities during lignocellulose degradation byStreptomyces sp.: a comparative study of wild-type and genetically manipulated strains.Appl.Environ.Microbiol.53, 2754–2760 (1987).
Reid I.D., Paice M.G.: Effects of manganese peroxidase on residual lignin of softwood kraft pulp.Appl.Environ.Microbiol.64, 2273–2274 (1998).
Rob A., Ball A.S., Tuncer M., Wilson M.T.: Thermostable novel non-hem extracellular glycosylated peroxidase fromThermomonospora fusca BD25.Biotechnol.Appl.Biochem.24, 161–170 (1996).
Schwarz W.H., Bronnenmeier K., Krause B., Lottspeich F., Staudenbauer W.L.: Debranching of arabinoxylan: properties of the thermoactive recombinant α-l-arabinofuranosidase fromClostridium stercorarium (arfB).Appl.Microbiol.Biotechnol.43, 856–860 (1995).
Senior D.J., Hamilton J., Bernier R.L. Jr.: Use ofStreptomyces lividans xylanase for bleaching of kraft pulps, pp. 555–558 in J. Visser, G. Beldman, M.A. Kusters van Somerenand, A.G.J. Voragen (Eds):Xylans and Xylanases. Progress in Biotechnology, Vol. 7. Elsevier Applied Science, Amsterdam (The Netherlands) 1992.
Tagawa K., Kaji A.: α-l-Arabinofuranosidase fromAspergillus niger.Meth.Enzymol.160, 707–712 (1988).
Tajana E., Fiechter A., Zimmermann W.: Purification and characterization of two α-l-arabinofuranosidases fromStreptomyces diastaticus.Appl.Environ.Microbiol.58, 1447–1450 (1992).
Thomson J.A.: Molecular biology of xylan degradation.FEMS Microbiol.Rev.104, 65–82 (1993).
Trigo C., Ball A.S.: Production of extracellular enzymes during the solubilization of straw byThermomonospora fasca BD25.Appl.Microbiol.Biotechnol.41, 366–372 (1994).
Tuncer M.: Characterization of β-xylosidase and α-l-arbinofuranosidase activities fromThermomonospora fusca BD25.Turk.J.Biol.24, 753–767 (2000).
Tuncer M., Rob A., Ball A.S., Wilson M.T.: Production of extracellular lignocellulose degrading enzymes byThermomonospora fusca BD25.Biochem.Soc.Trans.24, S378 (1996).
Tuncer M., Rob A., Ball A.S., Eady R.R., Henderson N., Wilson M.T.: Optimization of production of extracellular non-hem peroxidases byThermomonospora fusca BD25 in aerobic bio-reactor conditions.Biochem.Soc.Trans.25, S65 (1997).
Tuncer M., Rob A., Ball A.S., Wilson M.T.: Optimization of extracellular lignocellulolytic enzyme production by a thermophilic actinomyceteThermomonospora fusca BD25.Enzyme Microbiol.Technol.25, 38–47 (1999).
Utt E.A., Eddy C.K., Keshav K.F., Ingram L.O.: Sequencing and expression of theButyrivibrio fibrisolvens xylB gene encoding a novel bifunctional protein with β-d-xylosidase and α-l-arabinofuranosidase activities.Appl.Environ.Microbiol.57, 1227–1234 (1991).
Viikari L., Kantelinen A., Sundquist J., Linko M.: Xylanases in bleaching: from an idea to the industry.FEMS Microbiol.Rev.13, 335–350 (1994).
Wood T.M., McCrae S.I.: Arabinoxylan-degrading enzyme system of the fungusAspergillus awamori: purification and properties of an α-l-arabinofuranosidase.Appl.Microbiol.Biotechnol.45, 538–545 (1996).
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was carried out in the frame of a postgraduate studentship provided for the first author byUniversity of Essex (UK); the first author also acknowledges the scholarship provided byMersin University (Turkey).
Rights and permissions
About this article
Cite this article
Tuncer, M., Ball, A.S. Purification and partial characterization of α-l-arabinofuranosidase produced byThermomonospora fusca . Folia Microbiol 48, 168–172 (2003). https://doi.org/10.1007/BF02930950
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02930950