Abstract
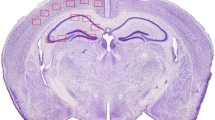
Transgenic mice overexpressing the 770-amino acid isoform of human Alzheimer amyloid precursor protein exhibit extracellular β-amyloid deposits in brain regions including cerebral cortex and hippocampus, which are severely affected in Alzheimer’s disease patients. Significant reduction in choline acetyltransferase (ChAT) activities has been observed in both cortical and hippocampal brain regions in the transgenic mice at the age of 10 months compared with the age-matched non-transgenic mice, but such changes have not been observed in any brain regions of the transgenic mice under the age of 5 months. These results suggest that deposition of β-amyloid can induce changes in the brain cholinergic system of the transgenic mice.
Similar content being viewed by others
References
Price, D. L., Borchelt, D. R., Sisodia, S. S., Alzheimer disease and the prion disorders: amyloid β-protein and prion protein amyloidoses, Proc. Natl. Acad. Sci. USA, 1993, 90: 6381.
Davies, P., Maloney, A. J. F., Selective loss of central cholinergic neurons in Alzheimer’s disease, Lancet, 1976, ii: 1403.
Selkoe, D. J., Normal and abnormal biology of the beta-amyloid precursor protein, Ann. Rev. Neurosci., 1994, 17: 489.
Kidd, M., Paired helical filaments in electron microscopy of Alzheimer’s disease, Nature, 1963, 197: 192.
Goedert, M., Wischik, C. M., Crowther, R. A. et al., Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer’s disease: identification as the microtubule-associated protein tau, Proc. Natl. Acad. Sci. USA, 1988, 85: 4051.
Stieber, A., Mourelatos, Z., Gonates, N. K., In Alzheimer’s disease the Golgi apparatus of a population on neurons without neurofribrillary tangles is fragmented and atrophic, American Journal of Pathology, 1996, 148: 415.
Jung, L. J., Scheller, R. H., Peptide processing and targeting in the neuronal secretory pathway, Science, 1991, 231: 1330.
Tanaka, S., Nakamura, S., Ueda, K. et al., Three types of amyloid protein precursor mRNA in human brain: their differential expression in Alzheimer’s disease, Biochem. Biophys. Res. Commun., 1988, 157: 472.
Citron, M., Olterdorf, T., Haass, C. et al., Mutation of the b-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production, Nature, 1992, 360: 672.
Scheuner, D., Eckman, C., Jensen, M. et al., Secreted amyloid b-protein similar to that in the senile plaques of Alzheimer’s disease is increasedin vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease, Nature Medicine, 1996, 2: 864.
Saunders, A. M., Strttermatter, W. J., Schmechel, D., Association of apolipoprotein E allele e4 with late-onset familial and sporadic Alzheimer’s disease, Neurology, 1993, 43: 1467.
Cotman, C. W., Pike, C. J., Copani, A., β-amyloid neurotoxity: a discussion ofin vitro findings, Neurobiol. Aging, 1992, 13: 587.
Nitta, A., Itoh, A., Hasegawa, T., β-amyloid protein-induced Alzheimer’s disease animal model, Neuroscience Letter, 1994, 170: 63.
Hogan, B., Costantini, F., Lacy, E., Manipulating the Mouse Embryo, A Laboratory Manual, New York: Cold Spring Harbor, 1986.
Tamaoka, A., Kalaria, R. N., Lieberburg, I., Identification of a stable fragment of the Alzheimer amyloid precursor containing the β-protein in brain microvessels, Proc. Natl. Acad. Sci. USA, 1992, 89: 1345.
Fonnum, F., A rapid radiochemical method of determination of choline acetyltransferase, Journal of Neurochemistry, 1975, 24: 407.
Ali, S. M., Siedlak, S. L., Gonzalez-DeWhitt, P. A. et al., Artifactual strain-specific signs of incipient brain amyloid in APP transgenic mice, Neurobiology of Aging, 1996, 17: 223.
Li, H., Chan, S. T. H., Tang, F., Transfection of rat brain cells by electroporation, Journal of Neuroscience Methods, 1997, 75: 29.
Quon, D., Wang, Y., Catalano, R. et al., Formation of β-amyloid protein deposits in brains of transgenic mice, Nature, 1991, 352: 239.
Games, D., Adams, D., Alessandrini, R. et al., Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein, Nature, 1995, 373: 523.
Hsiao, K., Chapman, P., Nilson, S. et al., Correlative memory deficits, Aβ elevation and amyloid plaques in transgenic mice, Science, 1996, 274: 99.
Sturchler-Pierrat, C., Abramowski, D., Duke, M. et al., Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology, Proc. Natl. Acad. Sci. USA, 1996, 94: 13287.
Author information
Authors and Affiliations
About this article
Cite this article
Li, H. Reduction of choline acetyltransferase activities inAPP 770 transgenic mice. Chin.Sci.Bull. 45, 834–838 (2000). https://doi.org/10.1007/BF02887413
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02887413