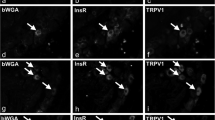
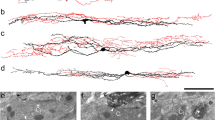
Abstract
Primary sensory neurons serve a dual role as afferent neurons, conveying sensory information from the periphery to the central nervous system, and as efferent effectors mediating, e.g., neurogenic inflammation. Neuropeptides are crucial for both these mechanisms in primary sensory neurons. In afferent functions, they act as messengers and modulators in addition to a principal transmitter; by release from peripheral terminals, they induce an efferent response, “neurogenic inflammation,” which comprises vasodilatation, plasma extravasation, and recruitment of immune cells. In this article, we introduce two novel members of the sensory neuropeptide family: pituitary adenylate cyclase-activating polypeptide (PACAP) and islet amyloid polypeptide (IAPP). Whereas PACAP, a vasoactive intestinal polypeptide-resembling peptide, predominantly occurs in neuronal elements, IAPP, which is structurally related to calcitonin gene-related peptide, is most widely known as a pancreatic β-cell peptide; as such, it has been recognized as a constituent of amyloid deposits in type 2 diabetes. In primary sensory neurons, under normal conditions, both peptides are predominantly expressed in small-sized nerve cell bodies, suggesting a role in nociception. On axotomy, the expression of PACAP is rapidly induced, whereas that of IAPP is reduced. Such a regulation of PACAP suggests that it serves a protective role during nerve injury, but that of IAPP may indicate that it is an excitatory messenger under normal conditions. In contrast, in localized adjuvant-induced inflammation, expression of both peptides is rapidly induced. For IAPP, studies in IAPP-deficient mice support the notion that IAPP is a pronociceptive peptide, because these mutant mice display a reduced nociceptive response when challenged with formalin.
Similar content being viewed by others
References
Aiyar N., Rand K., Elshourbagy N. A., Zeng Z., Adamou J. E., Bergsma D. J., et al. (1996) A cDNA encoding the calcitonin gene-related peptide type 1 receptor.J. Biol. Chem. 271, 11,325–11,329.
Aldskogius H., Arvidsson J., and Grant G. (1985) The reaction of primary sensory neurons to peripheral nerve injury with particular emphasis on transganlionic changes.Brain Res. 357, 27–46.
Amara S. G., Arriza J. L., Leff S. E., Swanson L. W., Evans R. M., and Rosenfeld M. G. (1985) Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide.Science 229, 1094–1097.
Arimura A., Somogyvari-Vigh A., Weill C., Fiore R. C., Tatsuno I., Bay V., et al. (1994) PACAP functions as a neurotrophic factorAnn. NY Acad. Sci. 739, 228–243.
Ar'Rajab A., and Ahrén B. (1991) Effects of amidated rat islet amyloid polypeptide on glucose-stimulated insulin secretion in vivo and in vitro in rats.Eur. J. Pharmacol. 192, 443–445.
Barnes P. J., Belvisi M. G., and Rogers D. F. (1990) Modulation of neurogenic inflammation: novel approaches to inflammatory disease.Trends Pharmacol. Sci. 11, 185–189.
Beaumont K., Kenney M. A., Young A. A., and Rink T. J. (1993) High affinity amylin binding sites in rat brain.Mol. Pharmacol. 44, 493–497.
Brain S. D. and Williams T. J. (1988) Substance P regulates the vasodilator activity of calcitonin gene-related peptide.Nature 335, 73–75.
Brain S. D., Williams T. J., Tippins J. R., Morris H. R., and MacIntyre I. (1985) Calcitonin generelated peptide is a potent vasodilator.Nature 313, 54–56.
Brain S. D., Wimalawansa S., MacIntyre I., and Williams T. J. (1990) The demonstration of vasodilator activity of pancreatic amylin amide in the rabbit.Am. J. Pathol. 136, 487–490.
Cao Y. Q., Mantyh P. W., Carlson E. J., Gillespie A. M., Epstein C. J., and Basbaum A. I. (1998) Primary afferent tachykinins are required to experience moderate to intense pain.Nature 392, 390–394.
Cardell L. O., Uddman R., Luts A., and Sundler F. (1991) Pituitary adenylate cyclase activating peptide (PACAP) in guinea-pig lung: distribution and dilatory effectsRegul. Pept. 36, 379–390.
Chin S. Y., Hall J. M., Brain S. D., and Morton I. K. (1994) Vasodilator responses to calcitonin generelated peptide (CGRP) and amylin in the rat isolated perfused kidney are mediated via CGRP1 receptors.J. Pharmacol. Exp. Ther. 269, 989–992.
Clark A., Chargé S. B. P., Badman M. K., MacArthur D. A., and de Koning E. J. P. (1996) Islet amyloid polypeptide: Actions and role in the pathogenesis of diabetes,Biochem. Soc. Trans. 24, 594–599.
Clementi G., Caruso A., Cutuli V. M. C., Prato A., Debernardis E., Fiore C. E., et al. (1995) Antiinflammatory activity of amylin and CGRP in different experimental models of inflammation.Life Sci. 57, PL193-PL197.
Cooper G. J. S. (1994) Amylin compared with calcitonin gene-related peptide—structure, biology, and relevance to metabolic disease.Endocr. Rev. 15, 163–201.
Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., and Reid K. B. (1987) Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients.Proc. Natl. Acad. Sci. USA 84, 8628–8632.
Dagerlind Å., Friberg K., Bean A. J., and Hökfelt T. (1992) Sensitive mRNA detection using unfixed tissue: combined radioactive and non-radioactive in situ hybridization histochemistry.Histochemistry 98, 39–49.
Danielsen N., Lundborg G., and Frizell M. (1986) Nerve repair and axonal transport: outgrowth delay and regeneration rate after transection and repair of rabbit hypoglossal nerve.Brain Res. 376, 125–132.
Dickinson T., Fleetwood Walker S. M., Mitchell R., and Lutz E. M. (1997) Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs.Neuropeptides 31, 175–185.
Donaldson L. F., Harmar A. J., McQueen D. S., and Seckl J. R. (1992) Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat,Mol. Brain Res. 16, 143–149.
Donaldson L. F., Seckl J. R., and McQueen D. S. (1993) A discrete adjuvant-induced monoarthritis in the rat: effects of adjuvant dose.J. Neurosci. Methods. 49, 5–10.
Donnerer J., Schuligoi R., and Stein C. (1992) Increased content and transport of substance P and calcitonin gene-related peptide in sensory nerves innervating inflamed tissue: evidence for a regulatory function of nerve growth factor in vivo.Neuroscience 49, 693–698.
Doughty S. E., Atkinson M. E., and Shehab S. A. (1991) A quantitative study of neuropeptide immunoreactive cell bodies of primary afferent sensory neurons following rat sciatic nerve peripheral axotomy.Regul. Pept. 35, 59–72.
Dun E. C., Huang R. L., Dun S. L., and Dun N. J. (1996) Pituitary adenylate cyclase activating polypeptide-immunoreactivity in human spinal cord and dorsal root ganglia.Brain Res. 721, 233–237.
Ferrier G. J., Pierson A. M., Jones P. M., Bloom S. R., Girgis S. I., and Legon S. (1989) Expression of the rat amylin (IAPP/DAP) gene.J. Mol. Endocrinol. 3, R1-R4.
Filipsson K., Tornoe K., Holst J. J., and Ahrén B. (1997) Pituitary adenylate cyclase-activating polypeptide stimulates insulin and glucagon secretion in humans.J. Clin. Endocrinol. Metab. 82, 3093–3098.
Filipsson K., Pacini G., Scheurink A. J., and Ahrén B. (1998) PACAP stimulates insulin secretion but inhibits insulin sensitivity in mice.Am. J. Physiol. 274, E834-E842.
Fitzgerald M., Wall P. D., Goedert M., and Emson P. C. (1985) Nerve growth factor counteracts the neurophysiological and neurochemical effects of chronic sciatic nerve section.Brain Res. 332, 131–141.
Fridolf T., Sundler F., and Ahrén B. (1992) Pituitary adenylate cyclase-activating polypeptide (PACAP): occurrence in rodent pancreas and effects on insulin and glucagon secretion in the mouse.Cell Tissue Res. 269, 275–279.
Frodin M., Hannibal J., Wulff B. S., Gammeltoft S., and Fahrenkrug J. (1995) Neuronal localization of pituitary adenylate cyclase-activating polypeptide 38 in the adrenal medulla and growth-inhibitory effect on chromaffin cells.Neuroscience 65, 599–608.
Garrett N. E., Kidd B. L., Cruwys S. C., and Tomlinson D. R. (1995) Changes in preprotachykinin mRNA expression and substance P levels in dorsal root ganglia of monoarthritic rats: comparison with changes in synovial substance P levels.Brain Res. 675, 203–207.
Gebre-Medhin S., Mulder H., Pekny M., Westermark G., Törnell J., Westermark P., et al. (1998a) Increased insulin secretion and glucose tolerance in mice lacking islet amyloid polypeptide (amylin).Biochem. Biophys. Res. Commun. 250, 271–277.
Gebre-Medhin S., Mulder H., Zhang Y., Sundler F., and Betsholtz C. (1998b) Reduced nociceptive behavior in islet amyloid polypeptide (amylin) knock out mice.Mol. Brain Res. 63, 180–183.
Ghatei M. A., Takahashi K., Suzuki Y., Gardiner J., Jones P. M., and Bloom S. R. (1993) Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues.J. Endocrinol. 136, 159–166.
Grafstein B. and Forman D. S. (1980) Intracellular transport in neurons.Physiol. Rev. 60, 1167–1283.
Gras S., Hannibal J., Georg B., and Fahrenkrug J. (1996) Transient periovulatory expression of pituitary adenylate cyclase activating peptide in rat ovarian cells.Endocrinology 137, 4779–4785.
Hanesch U., Pfrommer U., Grubb B. D., and Schaible H. G. (1993) Acute and chronic phases of unilateral inflammation in rat's ankle are associated with an increase in the proportion of calcitonin gene-related peptide-immunoreactive dorsal root ganglion cells.Eur. J. Neurosci. 5, 154–161.
Hannibal J. and Fahrenkrug J. (1995) Expression of pituitary adenylate cyclase activating polypeptide (PACAP) gene by rat spermatogenic cells.Regul. Pept. 55, 111–115.
Hannibal J., Mikkelsen J. D., Fahrenkrug J., and Larsen P. J. (1995) Pituitary adenylate cyclase-activating peptide gene expression in corticotropin-releasing factor-containing parvicellular neurons of the rat hypothalamic paraventricular nucleus is induced by colchicine, but not by adrenalectomy, acute osmotic, ether, or restraint stress.Endocrinology 136, 4116–4124.
Hannibal J., Ekblad E., Mulder H., Sundler F., and Fahrenkrug J. (1998) Pituitary adenylate cyclase activating polypeptide (PACAP) in the gastrointestinal tract of the rat: distribution and effects of capsaicin or denervation.Cell Tissue Res. 291, 65–79.
Harmar A. J., Arimura A., Gozes I., Journot L., LaBurthe M., Pisegna J. R., et al. (1998) International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating poypeptide.Pharmacol. Rev. 50, 265–270.
Helme R. D., Koschorke G. M., and Zimmermann M. (1986) Immunoreactive substance P release from skin nerves in the rat by noxious thermal stimulation.Neurosci. Lett. 63, 295–299.
Henken D. B., Battisti W. P., Chesselet M. F., Murray M., and Tessler A. (1990) Expression of beta-preprotachykinin mRNA and tachykinins in rat dorsal root ganglion cells following peripheral or central axotomy.Neuroscience 39, 733–742.
Heumann R., Korsching S., Bandtlow, C., and Thoenen H. (1987) Changes of nerve growth factor synthesis in nonneuronal cells in response to sciatic nerve transection.J. Cell Biol. 104, 1623–1631.
Himes B. T. and Tessler A. (1989) Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats.J. Comp. Neurol. 284, 215–230.
Holzer P. (1988) Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides.Neuroscience 24, 739–768.
Hom J. T., Estridge T., Pechous P., and Hyslop P. A. (1995) The amyloidogenic peptide human amylin augments the inflammatory activities of eostinophils.J. Leukocyte Biol. 58, 526–532.
Hökfelt T. (1991) Neuropeptides in perspective: the last ten years.Neuron 7, 867–879.
Hökfelt T., Zhang X., and Wiesenfeld-Hallin Z. (1994) Messenger plasticity in primary sensory neurons following axotomy and its functional implications.Trends Neurosci.17, 22–30.
Jessell T., Tsunoo A., Kanazawa I., and Otsuka M. (1979) Substance P: depletion in the dorsal horn of rat spinal cord after section of the peripheral processes of primary sensory neurons.Brain Res. 168, 247–259.
Johnson K. H., O'Brien T. D., Hayden D. W., Jordan K., Ghobrial H. K., Mahoney W. C., et al. (1988) Immunolocalization of islet amyloid polypeptide (IAPP) in pancreatic beta cells by means of peroxidase-antiperoxidase (PAP) and protein A-gold techniques.Am. J. Pathol. 130, 1–8.
Jongsma H., Danielsen N., Sundler F., and Kanje M. (1998) Alterations of PACAP and PACAP receptor binding in sensory neurons following peripheral axotomy.Soc. Neurosci. Abstract 24, 2049.
Kapas S. and Clark A. J. (1995) Identification of an orphan receptor gene as a type 1 calcitonin generelated peptide receptor.Biochem. Biophys. Res. Commun. 217, 832–838.
Kawaguchi Y., Hoshimaru M., Nawa H., and Nakanishi S. (1986) Sequence analysis of cloned cDNA for rat substance P precursor: existence of a third substance P precursor.Biochem. Biophys. Res. Commun. 139, 1040–1046.
Korsching S. and Thoenen H. (1983) Quantitative demonstration of the retrograde axonal transport of endogenous nerve growth factor.Neurosci. Lett. 39, 1–4.
Köves K., Arimura A., Vigh S., Somogyvari-Vigh A., and Miller J. (1993) Immunohistochemical localization of PACAP in the ovine digestive system.Peptides 14, 449–455.
Krause J. E., Chirgwin J. M., Carter M. S., Xu Z. S., and Hershey A. D. (1987) Three rat preprotachykinin mRNAs encode the neuropeptides substance P and neurokinin A.Proc. Natl. Acad. Sci. USA 84, 881–885.
Larson A. A., Brown D. R., el Atrash S., and Walser M. M. (1986) Pain threshold changes in adjuvant-induced inflammation: a possible model of chronic pain in the mouse.Pharmacol. Biochem. Behav. 24, 49–53.
Leighton B. and Cooper G. J. (1988) Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro.Nature 335, 632–635.
Lieberman A. R. (1971) The axon reaction: a review of the principal features of perikaryal responses to axon injury.Int. Rev. Neurobiol. 14, 49–124.
Lindsay R. M. and Harmar A. J. (1989) Nerve growth genes in adult sensory neurons.Nature 337, 362–364.
Lioudyno M., Skoglösa Y., Takei N., and Lindholm D. (1998) Pituitary adenylate cyclase-activating polypeptide (PACAP) protects dorsal root ganglion neurons from death and induces calcitonin gene-related peptide (CGRP) immunoreactivity in vitro.J. Neurosci. Res. 51, 243–256.
Lutz E. M., Mendelson S., West K., Mitchell R., and Harmar A. J. (1995) Molecular characterisation of novel receptors for PACAP and VIP.Biochem. Soc. Trans. 23, 83S.
Mackinnon S. E. and Dellon A. L. (1988) Painful sequelae of peripheral nerve injur, inSurgery of the Peripheral Nerve. Thieme. Medical Publishers, New York, pp. 455–519.
Maggi C. A. and Meli A. (1988) The sensory-efferent function of capsaicin-sensitive sensory neurons.Gen. Pharmacol. 19, 1–43.
Millan M. J., Czlonkowski A., Morris B., Stein C., Arendt R., Huber A., et al. (1988) Inflammation of the hind limb as a model of unilateral, localized pain: influence on multiple opioid systems in the spinal cord of the rat.Pain 35, 299–312.
Miyata A., Arimura A., Dahl R. R., Minamino N., Uehara A., Jiang L., et al. (1989) Isolation of a novel PACAP-38 residue-hypothalamic peptide which stimulates adenylate cyclase in pituitary cells.Biochem. Biophys. Res. Commun. 164, 567–574.
Miyata A., Jiang L., Dahl R. R., Kitada C., Kubo K., Fujino M., et al. (1990) Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP-38).Biochem. Biophys. Res. Commun. 170, 643–648.
Moller K., Zhang Y. Z., Håkanson R., Luts A., Sjölund B., Uddman R., et al. (1993) Pituitary adenylate cyclase activating peptide is a sensory neuropeptide: immunocytochemical and immunochemical evidence.Neuroscience 57, 725–732.
Moller K., Reimer M., Ekblad E., Hanibal J., Fahrenkrug J., Kanje M., et al. (1997a) The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion.Brain Res. 775, 166–182.
Moller K., Reimer M., Hannibal J., Fahrenkrug J., Sundler F., and Kanje M. (1997b) Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro.Brain Res. 775, 156–165.
Moroo I., Tatsuno I., Uchida D., Tanaka T., Saito J., Saito Y., et al. (1998) Pituitary adenylate cyclase activating polypeptide (PACAP) stimulates mitogen-activated protein kinase (MAPK) in cultured rat astrocytes.Brain Res. 795, 191–196.
Mulder H., Lindh A. C., and Sundler F. (1993) Islet amyloid polypeptide gene expression in the endocrine pancreas of the rat: a combined in situ hybridization and immunocytochemical study.Cell Tissue Res. 274, 467–474.
Mulder H., Uddman R., Moller K., Zhang Y. Z., Ekblad E., Alumets J., et al. (1994) Pituitary adenylate cyclase activating polypeptide expression in sensory neurons.Neuroscience 63, 307–312.
Mulder H., Leckström A., Uddman R., Ekblad E., Westermark P., and Sundler F. (1995a) Islet amyloid polypeptide (amylin) is expressed in sensory neurons.J. Neurosci. 15, 7625–7632.
Mulder H., Uddman R., Moller K., Elsås T., Ekblad E., Alumets J., et al. (1995b) Pituitary adenylate cyclase activating polypeptide is expressed in autonomic neurons.Regul. Pept. 59, 121–128.
Mulder H., Ekelund M., Ekblad E., and Sundler F. (1997a) Islet amyloid polypeptide in the gut and pancreas: localization, ontogeny and gut motility effects.Peptides 18, 771–783.
Mulder H., Zhang Y., Danielsen N., and Sundler F. (1997b) Islet amyloid polypeptide and calcitonin gene-related peptide expression are downregulated in dorsal root ganglia upon sciatic nerve transectionMol. Brain Res. 47, 322–330.
Mulder H., Zhang Y., Danielsen N., and Sundler F. (1997c) Islet amyloid polypeptide and calcitonin gene-related peptide expression are upregulated in lumbar dorsal root ganglia after unilateral adjuvant-induced inflammation in the rat paw.Mol. Brain Res. 50, 127–135.
Mulderry P. K., Ghatei M. A., Spokes R. A., Jones P. M., Pierson A. M., Hamid Q. A., et al. (1988) Differential expression of α-CGRP and β-CGRP by primary sensory neurons and enteric autonomic neurons of the rat.Neuroscience 25, 195–205.
Narita M., Dun S. L., Dun N. J., and Tseng L. F. (1996) Hyperalgesia induced by pituitary adenylate cyclase-activating polypeptide in the mouse spinal cord.Eur. J. Pharmacol. 311, 121–126.
Nicholl C. G., Bhatavdekar J. M., Mak J., Girgis S. I., and Legon S. (1992) Extra-pancreatic expression of the rat islet amyloid polypeptide (amylin) gene.J. Mol. Endocrinol. 9, 158–163.
Nielsen H. S., Hannibal J., and Fahrenkrug J. (1998) Embryonic expression of pituitary adenylate cyclase-activating polypeptide in sensory and autonomic ganglia and in spinal cord of the rat.J. Comp. Neurol. 394, 403–415.
Nishi M., Sanke T., Seino S., Eddy R. L., Fan Y. S., Byers M. G., et al. (1989) Human islet amyloid polypeptide gene: complete nucleotide sequence, chromosomal localization, and evolutionary history.Mol. Endocrinol. 3, 1775–1781.
Noguchi K., Morita Y., Kiyama H., Ono K., and Tohyama M. (1988) A noxious stimulus induces the preprotachykinin-A gene expression in the rat dorsal root ganglion: a quantitative study using in situ hybridization histochemistry.Brain Res. 464, 31–35.
Noguchi K., Senba E., Morita Y., Sato M., and Tohyama M. (1989) Prepro-VIP and preprotachykinin mRNAs in the rat dorsal root ganglion cells following peripheral axotomy.Mol. Brain Res. 6, 327–330.
Noguchi K., Senba E., Morita Y., Sato M., and Tohyama M. (1990) α-CGRP and β-CGRP mRNAs are differentially regulated in the rat spinal cord and dorsal root ganglion.Mol. Brain Res. 7, 299–304.
Noguchi K., De Leon M., Nahin R. L., Senba E., and Ruda M. A. (1993) Quantification of axotomy-induced alteration of neuropeptide mRNAs in dorsal root ganglion neurons with special reference to neuropeptide Y mRNA and the effects of neonatal capsaicin treatment.J. Neurosci. Res. 35, 54–66.
Nothias F., Tessler A., and Murray M. (1993) Restoration of substance P and calcitonin gene-related peptide in dorsal root ganglia and dorsal horn after neonatal sciatic nerve lesion.J. Comp. Neurol. 334, 370–384.
Ogi K., Kimura C., Onda H., Arimura A., and Fujino M. (1990) Molecular cloning and characterization of cDNA for the precursor of rat pituitary adenylate cyclase activating polypeptide (PACAP).Biochem. Biophys. Res. Commun. 173, 1271–1279.
Opie E. L. (1901) On the relation of chronic interstitial pancreatitis to the islands of Langerhans and to diabetes mellitus.J. Exp. Med. 5, 397–428.
Percy A. J., Trainor D. A., Rittenhouse J., Phelps J., and Koda J. E. (1996) Development of sensitive immunoassays to detect amylin and amylin-like peptides in unextracted plasma.Clin. Chem. 42, 576–585.
Pincus D. W., DiCicco Bloom E., and Black I. B. (1994) Trophic mechanisms regulate mitotic neuronal precursors: role of vasoactive intestinal peptide (VIP).Brain Res. 663, 51–60.
Poyner D. (1995) Pharmacology of receptors for calcitonin gene-related peptide and amylin.Trends Pharmacol. Sci. 16, 424–428.
Przywara D. A., Guo X., Angelilli M. L., Wakade T. D., and Wakade A. R. (1996) A non-cholinergic transmitter, pituitary adenylate cyclase-activating polypeptide, utilizes a novel mechanism to evoke catecholamine secretion in rat adrenal chromaffin cells.J. Biol. Chem. 271, 10,545–10,550.
Rawlings S. R. and Hezareh M. (1996) Pituitary adenylate cyclase-activating polypeptide (PACAP) and PACAP/vasoactive intestinal polypeptide receptors: actions on the anterior pituitary gland.Endocr. Rev. 17, 4–29.
Reimer M., Moller K., Sundler F., Hannibal J., Fahrenkrug J., and Kanje M. (1998) Increased expression, axonal transport and release of pituitary adenylate cyclase-activating polypeptide in the cultured rat vagus nerve.Neuroscience 88, 213–222.
Salt T. E. and Hill R. G. (1983) Neurotransmitter candidates of somatosensory primary afferent fibres.Neuroscience 10, 1083–1103.
Schwartz J. P. (1992) Neurotransmitters as neurotrophic factors: a new set of functions.Int. Rev. Neurobiol. 34, 1–23.
Shen Z., Larsson L. T., Malmfors G., Absood A., Håkanson R., and Sundler F. (1992) A novel neuropeptide, pituitary adenylate cyclase-activating polypeptide (PACAP), in human intestine: evidence for reduced content in Hirschsprung's disease.Cell Tissue Res. 269, 369–374.
Sheward W. J., Lutz E. M., Copp A. J., and Harmar A. J. (1998) Expression of PACAP, and PACAP type 1 (PAC1) receptor mRNA during development of the mouse embryo.Dev. Brain Res. 109, 245–253.
Shioda S., Legradi G., Leung W.-C., Nakajo S., Nakaya K., and Arimura A. (1994) Localization of pituitary adenylate cyclase-activating polypeptide and its messenger ribonucleic acid in the rat testis by light and electron microscopic immunocytochemistry and in situ hybridization.Endocrinology 135, 818–825.
Soeller W. C., Janson J., Hart S. E., Parker J. C., Carty M. D., Stevenson R. W., et al. (1998) Islet amyloid-associated diabetes in obese Avy/a mice expressing human islet amyloid polypeptide.Diabetes 47, 743–750.
Steenstrup B. R., Alm P., Hannibal J., Jorgensen J. C., Palle C., Junge J., et al. (1995) Pituitary adenylate cyclase-activating polypeptide: occurrence and relaxant effect in female genital tract.Am. J. Physiol. 1, E108-E117.
Sundler F., Brodin E., Ekblad E., Håkanson R., and Uddman R. (1985) Sensory nerve fibers: distribution of substance P, neurokinin A and calcitonin gene-related peptide, inTachykinin Antagonists (Håkanson R., and Sundler F., eds.), Elsevier Science, Amsterdam, pp. 3–14.
Sundler F., Ekblad E., Absood A., Håkanson R., Köves K., and Arimura A. (1992) Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut.Neuroscience 46, 439–454.
Sundler F., Ekblad E., Hannibal J., Moller K., Zhang Y. Z., Mulder H., et al. (1996) Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation.Ann. N Y Acad. Sci. 805, 410–426.
Szolcsanyi J. (1988) Antidromic vasodilatation and neurogenic inflammation.Agents Actions 23, 4–11.
Tessler A., Himes B. T., Krieger N. R., Murray M., and Goldberger M. E. (1985) Sciatic nerve transection produces death of dorsal root ganglion cells and reversible loss of substance P in spinal cord.Brain Res. 332, 209–218.
Tjölsen A., Berge O. G., Hunskaar S., Rosland J. H., and Hole K. (1992) The formalin test: an evaluation of the method.Pain 51, 5–17.
Tobin G., Asztély A., Edwards A. V., Ekström J., Håkanson R., and Sundler F. (1994) Presence and effects of pituitary adenylate cyclase activating peptide (PACAP) in the submandibular gland of the ferret.Neuroscience 66, 227–235.
Uchida D., Arimura A., Somogyvari-Vigh A., Shioda S., and Banks W. A. (1996) Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide.Brain Res. 736, 280–286.
Uddman R., Edvinsson L., Ekblad E., Håkanson R., and Sundler F. (1986) Calcitonin gene-related peptide (CGRP): perivascular distribution and vasodilatory effects.Regul. Pept. 15, 1–23.
Uddman R., Luts A., Arimura A., and Sundler F. (1991) Pituitary adenylate cyclase-activating peptide (PACAP), a new vasoactive intestinal peptide (VIP)-like peptide in the respiratory tract.Cell Tissue Res. 265, 197–201.
Urabe T., Zhao Q., Lundborg G., and Danielsen N. (1995) Effects of delayed nerve repair on regeneration of rat sciatic nerve.Restor. Neurol. Neurosci. 9, 1–5.
Vaudry D., Gonzalez B. J., Basille M., Anouar Y., Fournier A., and Vaudry H. (1998) Pituitary adenylate cyclase-activating polypeptide stimulates both c-fos gene expression and cell survival in rat cerebellar granule neurons through activation of the protein kinase A pathway.Neuroscience 84, 801–812.
Veale P. R., Bhogal R., Morgan D. G. A., Smith D. M., and Bloom S. R. (1994) The presence of islet amyloid polypeptide/calcitonin gene-related peptide/salmon calcitonin binding sites in the rat nucleus accumbens.Eur. J. Pharmacol. 262, 133–141.
Verchere C. B., D'Alessio D. A., Palmiter R. D., Weir G. C., Bonner-Weir S., Baskin D. G., et al. (1996) Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic beta cell expression of human islet amyloid polypeptide.Proc. Natl. Acad. Sci. USA 93, 3492–3496.
Verge V. M., Wiesenfeld-Hallin Z., and Hökfelt T. (1993) Cholecystokinin in mammalian primary sensory neurons and spinal cord: in situ hybridization studies in rat and monkey.Eur. J. Neurosci. 5, 240–250.
Verge V. M., Richardson P. M., Wiesenfeld-Hallin Z., and Hökfelt T. (1995) Differential influence of nerve growth factor on neuropeptide expression in vivo: a novel role in peptide suppression in adult sensory neurons.J. Neurosci. 15, 2081–2096.
Villalba M., Bockaert J., and Journot L. (1997) Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway.J. Neurosci. 17, 83–90.
Villar M. J., Cortes R., Theodorsson E., Wiesenfeld-Hallin Z., Schalling M., Fahrenkrug J., et al. (1989) Neuropeptide expression in rat dorsal root ganglion cells and spinal cord after peripheral nerve injury with special reference to galanin.Neuroscience 33, 587–604.
Wang Z. Y., Alm P., and Håkanson R. (1995) Distribution and effects of pituitary adenylate cyclase-activating peptide in the rabbit eye.Neuroscience 69, 297–308.
Warren J. B., Larkin S. W., Coughlan M., Kajekar R., and Williams T. J. (1992) Pituitary adenylate cyclase activating polypeptide is a potent vasodilator and oedema potentiator in rabbit skin in vivo.Br. J. Pharmacol. 106, 331–334.
Waschek J. A., Casillas R. A., Nguyen T. B., DiCicco Bloom E. M., Carpenter E. M., and Rodriguez W. I. (1998) Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: potential role in patterning and neurogenesis.Proc. Natl. Acad. Sci. USA 95, 9602–9607.
Weihe E., Nohr D., Millan M. J., Stein C., Muller S., Gramsch C., et al. (1988) Peptide neuroanatomy of adjuvant-induced arthritic inflammation in rat.Agents Actions 25, 255–259.
West K. M., Shen S., Sheward W. J., Mackay M. E. H., Kilanowski E. M., Lutz E. M., et al. (1998) Generation of mice lacking a receptor for VIP and PACAP (the VPAC2 receptor).Soc. Neurosci. Abstract 24, 2049.
Westermark P. (1973) Fine structure of islets of Langerhans in insular amyloidosis.Virchows Arch. A. Pathol. Anat. Histopathol. 359, 1–18.
Westermark P. (1994) Amyloid and polypeptide hormones: what is their inter-relationship?Amyloid: Int. J. Exp. Clin. Invest. 1, 47–60.
Westermark P., Wernstedt C., Wilander E., and Sletten K. (1986) A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas.Biochem. Biophys. Res. Commun. 140, 827–831.
Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., and Johnson K. H. (1987) Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells.Proc. Natl. Acad. Sci. USA 84, 3881–3885.
Westermark P., Engstrom U., Johnson K. H., Westermark G. T., and Betsholtz C. (1990) Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation.Proc. Natl. Acad. Sci. USA 87, 5036–5040.
Westermark P., Johnson K. H., O'Brien T. D., and Betsholtz C. (1992) Islet amyloid polypeptide—a novel controversy in diabetes research.Diabetologia 35, 297–303.
Xu X. J. and Wiesenfeld-Hallin Z. (1996) Intrathecal pituitary adenylate cyclase activating polypeptide facilitates the spinal nociceptive flexor reflex in the rat.Neuroscience 72, 801–804.
Yada T., Sakurada M., Ishihara H., Nakata M., Shioda S., Yaekura K., et al. (1997) Pituitary adenylate cyclase-activating polypeptide (PACAP) is an islet substance serving as an intra-islet amplifier of glucose-induced insulin secretion in rats.J. Physiol. (Lond.) 2, 319–328.
Yamamoto T. and Tatsuno I. (1995) Antinociceptive effect of intrathecally administered pituitary adenylate cyclase activating polypeptide (PACAP) on the rat formalin test.Neurosci. Lett. 184, 32–35.
Young A. (1997) Role of amylin in nutrient intake—animal studies.Diabetes Med. 2, S14-S18.
Zhang Q., Shi T.-J., Ji R.-R., Zhang Y.-Z., Sundler F., Hannibal J., et al. (1995) Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence.Brain Res. 705, 149–158.
Zhang X., Ju G., Elde R., and Hökfelt T. (1993) Effect of peripheral nerve cut on neuropeptides in dorsal root ganglia and the spinal cord of monkey with special reference to galanin.J. Neurocytol. 22, 342–381.
Zhang X., Åman K., and Hökfelt T. (1995) Secretory pathways of neuropeptides in rat lumbar dorsal root ganglion neurons and effects of peripheral axotomy.J. Comp. Neurol. 352, 481–500.
Zhang Y., Malmberg A. B., Sjölund B., and Yaksh T. L. (1996) The effect of pituitary adenylate cyclase activating peptide (PACAP) on the nociceptive formalin test.Neurosci. Lett. 207, 187–190.
Zhang Y., Danielsen N., Sundler F., and Mulder H. (1998) Pituitary adenylate cyclase-activating peptide is upregulated in sensory neurons by inflammation.Neuroreport 24, 2833–2836.
Zhang Y. Z., Sjölund B., Moller K., Håkanson R., and Sundler F. (1993) Pituitary adenylate cyclase activating peptide produces a marked and long-lasting depression of a C-fibre-evoked flexion reflex.Neuroscience 57, 733–737.
Zhang Y. Z., Hannibal J., Zhao Q., Moller K., Danielsen N., Fahrenkrug J., et al. (1996) Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury.Neuroscience 74, 1099–1110.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulder, H., Jongsma, H., Zhang, Y. et al. Pituitary adenylate cyclase-activating polypeptide and islet amyloid polypeptide in primary sensory neurons. Mol Neurobiol 19, 229–253 (1999). https://doi.org/10.1007/BF02821715
Issue Date:
DOI: https://doi.org/10.1007/BF02821715