Abstract
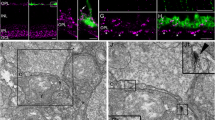
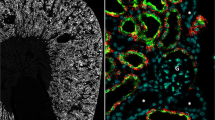
To investigate the relationship between the gap junction protein connexin 43 and the glucose transporter GLUT1, their localization was visualized by double-immunofluorescence microscopy using frozen sections as well as immunogold staining of ultrathin frozen sections. In pigmented epithelial cells, most of the GLUT1 was localized along the plasma membrane facing the blood vessels, whereas in non-pigmented epithelial cells. it was present along the plasma membrane facing the aqueous humor. Connexin 43 was abundant in the ciliary body and localized mainly in the gap junctions connecting the pigmented and non-pigmented epithelial cells. Localization of GLUT1 and connexin 43 in the blood-aqueous barrier suggests that GLUT1, connexin 43, and GLUT1 disposed in this order could be a machinery responsible for the transport of glucose across the blood-aqueous barrier.
Similar content being viewed by others
References
Bennett MVL, Barrio LC, Bargiello TA, Spray DC, Hertzberg E, Sáez JC (1991) Gap junctions: new tools, new answers, new questions. Neuron 6:305–320
Beyer EC, Paul DL, Goodenough DA (1987) Connexin 43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol 105:2621–2629
Coca-Prados M, Ghosh S, Gilula NB, Kumar NM (1992) Expression and cellular distribution of the α1 gap junction gene product in the ocular pigmented ciliary epithelium. Curr Eye Res 11:113–122
Dermietzel R, Spray DC (1993) Gap junctions in the brain: where, what type, how many and why? Trends Neurosci 16:186–192
Freddo TF (1987) Intercellular junctions of the ciliary epithelium in anterior uveitis. Invest Ophthalmol vis Sci 28:320–329
Goodenough DA, Musil LS (1993) Gap junctions and tissue business: problems and strategies for developing specific functional reagents. J Cell Sci Suppl 17:133–138
Harik SI, Kalaria RN, Andersson L, Lundahl P, Perry G (1990a) Immunocytochemical localization of the erythroid glucose transporter: abundance in tissue with barrier functions. J Neurosci 10:3862–3872
Harik SI, Kalaria RN, Whitney PM, Andersson L, Lundahl P, Ledbetter SR, Perry G (1990b) Glucose transporters are abundant in cells with ‘occluding” junctions at the blood-eye barriers. Proc Natl Acad Sci USA 87:4261–4264
Kogon M, Pappas GD (1975) Atypical gap junctions in the ciliary epithelium of the albino rabbit cye. J Cell Biol 66:671–676
Kuraoka A, Iida H, Hatae T, Shibata Y, Itoh M, Kurita T (1993) Localization of gap junction proteins, connexins 32 and 26, in rat and guinea pig liver as revealed by quick-freeze, deep-etch immunoelectron microscopy. J Histochem Cytochem 41:971–980
Loewenstein WR (1979) Junctional intercellular communication and the control of growth. Biochim Biophys Acta 560:1–65
Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF (1985) Sequence and structure of a human glucose transporter. Science 229:941–945
Musil LS, Cunningham BA, Edelman GM, Goodenough DA (1990) Differential phosphorylation of the gap junction protein connexin 43 in junctional communication-competent and-deficient cell lines. J Cell Biol 111:2077–2088
Raviola G (1974) Effects of paracentesis on the blood-aqueous barrier: an electron microscope study onMacaca mutatta using horseradish peroxidase as a tracer. Invest Ophthalmol 13:828–858
Raviola G (1977) The structural basis of the blood-ocular barriers. Exp Eye Res [Suppl] 25:27–53
Raviola G, Raviola E (1978) Intercellular junctions in the ciliary epithelium. Invest Ophthalmol Vis Sci 17:958–981
Shin B-C, Suzuki T, Matsuzaki T, Tanaka S, Kuraoka A, Shibata Y, Takata K (1996) Immunolocalization of GLUT1 and connexin 26 in the rat placenta. Cell Tissue Res (in press)
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol 38:87–93
Takata K, Singer SJ (1988) Phosphotyrosine-modified proteins are concentrated at the membranes of epithelial and endothelial cells during tissue development in chick embryos. J Cell Biol 106:1757–1764
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1990) Erythrocyte/HepG2-type glucose transporter is concentrated in cells of blood-tissue barriers. Biochem Biophys Res Commun 173:67–73
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1991a) Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in the ciliary body and iris of the rat eye. Invest Ophthalmol Vis Sci 32:1659–1666
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1991b) Localization of Na+-dependent active type and erythrocyte/HepG2-type glucose transporters in rat kidney; immuno-fluorescence and immunogold study. J Histochem Cytochem 39:287–298
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1992) Ultracytochemical localization of the erythrocyte/HepG2-type glucose transporter (GLUT1) in cells of the blood-retinal barrier in the rat. Invest Ophthalmol Vis Sci 33:377–383
Takata K, Kasahara M, Oka Y, Hirano H (1993) Mammalian sugar transporters: their localization and link to cellular functions. Acta Histochem Cytochem 26:165–178
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1994) Immunolocalization of glucose transporter GLUT1 in the rat placental barrier: possible role of GLUT1 and the gap junction in the transport of glucose across the placental barrier. Cell Tissue Res 276:411–418
Tokuyasu KT (1986) Application of cryoultramicrotomy to immunocytochemistry. J Microsc 143:139–149
Valnes K, Brandtzaeg P (1985) Retardation of immunofluorescence fading during microscopy. J Histochem Cytochem 33: 755–761
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shin, BC., Suzuki, T., Tanaka, S. et al. Connexin 43 and the glucose transporter, GLUT1, in the ciliary body of the rat. Histochem Cell Biol 106, 209–214 (1996). https://doi.org/10.1007/BF02484402
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02484402