Abstract
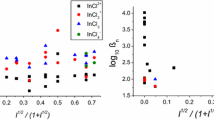
Titrations of Pu(IV) with HNO3 in a series of aqueous HClO4 solutions ranging in ionic strength from 2 to 19 molal were followed using visible and near-infrared absorption spectrophotometry. The Pu 5f-5f spectra in the visible and near IR range change with complex formation. At each ionic strength, a series of spectra were obtained by varying nitrate concentration. Each series was deconvoluted into spectra of Pu4+ (aq), Pu(NO3)3− and Pu(NO3)2 2+ complexes, and simultaneously their formation constants were determined. When corrected for the incomplete dissociation of nitric acid, the ionic strength dependence of each formation constant can be described by two parameters, β0 and Δε using the formulae of specific ion interaction theory.
Similar content being viewed by others
References
J. C. Hindman inG. T. Seaborg, J. J. Katz, W. M. Manning (Eds.), The Transuranium Elements: Research Papers, Vol. 14B, McGraw-Hill, New York 1949, p. 388.
J. A. Brothers, R. G. Hart, W. G. Mathers, J. Inorg. Nucl. Chem., 7 (1958) 85.
J. L. Ryan, J. Phys. Chem., 64 (1960), 1375.
J. M. Cleveland, Coord. Chem. Rev, 5 (1970) 101.
L. V. Lipis, B. G. Pozharskii, V. V. Fomin, Zhur. Strukt. Khim., 1 (1960) 135.
I. Grenthe, B. Noren, Acta Chem. Scand., 14 (1960) 2216.
T. S. Laxminarayanan, S. K. Patil, H. D. Sharma, J. Inorg. Nucl. Chem., 26 (1964) 1001.
P. R. Danesi, F. Orlandini, G. Scibona J. Inorg. Nucl. Chem., 28 (1966) 1047.
S. W. Rabidau, J. F. Lemons, J. Amer. Chem. Soc., 73 (1951) 2895.
H. S. Rossotti, Talanta, 21 (1974) 809.
M. Sampoli, A. D. Santis, N. C. Marziano, F. Pinna, A. Zingales, J. Phys. Chem., 89 (1985) 2864.
For a concise summary of SIT theory and additional references, see Appendix B of I. Grenthe, J. Fuger, R. J. M. Konngs, R. J. Lemire, A. B. Muller, C. Nguyen-Trung, H. Wanner Chemical Thermodynamics of Uranium, Elsevier Science Publishers, Amsterdam 1992.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Berg, J.M., Veirs, D.K., Vaughn, R.B. et al. Plutonium(IV) mononitrate and dinitrate complex formation in acid solutions as a function of ionic strength. J Radioanal Nucl Chem 235, 25–29 (1998). https://doi.org/10.1007/BF02385932
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02385932