Abstract
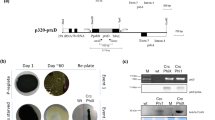
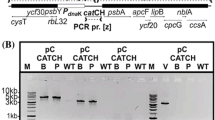
The bacterial geneaadA is an important and widely used selectable marker for manipulation of the chloroplast genome through biolistic transformation. Because no other such marker is available, two strategies for recycling of theaadA cassette have been developed. One utilizes homologous recombination between two direct repeats flanking theaadA cassette to allow its loss under non-selective growth conditions. A second strategy is to perform co-transformation with a plasmid containing a modified, non-essential chloroplast gene and another plasmid in which theaadA cassette disrupts a chloroplast gene known to be essential for survival. Under selective growth conditions the first mutation can be transferred to all chloroplast DNA copies whereas theaadA insertion remains heteroplasmic. Loss of the selectable marker can be achieved subsequently by growing the cells on non-selective media. In both cases it is possible to reuse theaadA cassette for the stepwise disruption or mutagenesis of any gene in the same strain.
Similar content being viewed by others
References
Alani E, Cao L, Kleckner N (1987) A method for gene disruption that allows repeated use ofURA3 selection in the construction of multiply disrupted yeast strains. Genetics 116:541–545
Bennoun P, Girard J, Chua N-H (1977) A uniparental mutant ofChlamydomonas reinhardtii deficient in the chlorophyll-protein complex CP1. Mol Gen Genet 153:343–348
Blowers AD, Bogorad L, Shark KB, Sanford JC (1989) Studies onChlamydomonas chloroplast transformation: foreign DNA can be stably maintained in the chromosome. The Plant Cell 1:123–132
Boynton JE, Gilham NW, Harris EH, Hosler JP, Johnson AM, Jones AR, Randolph-Anderson BL, Robertson D, Klein TM, Shark KB, Sanford JC (1988) Chloroplast transformation inChlamydomonas with high velocity microprojectiles. Science 240:1534–1538
Boynton JE, Gilham NW, Harris EH, Newman SM, Randolph-Anderson BL, Johnson AM, Jones AR (1990) Manipulating the chloroplast genome ofChlamydomonas: molecular genetics and transformation. Curr Res Photosynth 3:509–516
Boynton JE, Gillham NW, Newman SN, Harris EH (1992) Organelle genetics and transformation ofChlamydomonas. In: Herrmann RC (ed) Cell organelles (Advances in Plant Gene Research, vol. 6). Springer-Verlag, Vienna
Carrer H, Maliga P (1995) Targeted insertion of foreign genes into the tobacco plastid genome without physical linkage to the selectable marker gene. Bio Technology 13:791–794
Cerutti H, Johnson AM, Boynton JE, Gilham NW (1995) Inhibition of chloroplast DNA recombination and repair by dominant negative mutants ofEscherichia coli RecA. Mol Cell Biol 15:3003–3011
Chua N-H, Matlin K, Bennoun P (1975) A chlorophyll-protein complex lacking in photosystem I mutants ofChlamydomonas reinhardtii. J Cell Biol 67:361–377
Finer JJ, Vain P, Jones MW, McMullen MD (1992) Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep 11:323–328
Goldschmidt-Clermont M (1991) Transgenic expression of aminoglycoside adenine transferase in the chloroplast: a selectable marker for site-directed transformation ofChlamydomonas. Nucleic Acid Res 19:4083–4089
Harris EH (1989) TheChlamydomonas sourcebook. Academic Press, San Diego
Huang C, Wang S, Chen L, Lemieux C, Otis C, Turmel M, Liu XQ (1994) TheChlamydomonas chloroplastclpP gene contains translated large insertion sequences and is essential for cell growth. Mol Gen Genet 244:151–159
Kindle KL, Richards KL, Stern DB (1991) Engineering the chloroplast genome: techniques and capabilities for chloroplast transformation inChlamydomonas reinhardtii. Proc Natl Acad Sci USA 88:1721–1725
Künstner P, Guardiola A, Takahashi Y, Rochaix J-D (1995) A mutant strain ofChlamydomonas reinhartii lacking the chloroplast photosystem IIpsbI gene grows photoautotrophically. J Biol Chem 270:9651–9654
Newman SM, Boynton JE, Gilham NW, Randolph-Anderson BL, Johnson AM, Harris EH (1990) Transformation of chloroplast ribosomal RNA genes inChlamydomonas: molecular and genetic characterization of integration events. Genetics 126:875–888
Newman SM, Gilham NW, Harris EH, Johnson AM, Boynton JE (1991) Targeted disruption of chloroplast genes inChlamydomonas reinhardtii. Mol Gen Genet 230:65–74
Rochaix J-D (1978) Restriction endonuclease map of the chloroplast DNA ofChlamydomonas reinhardtii. J Mol Biol 126:597–617
Rochaix J-D, Mayfield S, Goldschmidt-Clermont M, Erickson J (1988) Molecular biology ofChlamydomonas. In: Shaw CH (ed) Plant molecular biology: a practical approach. IRL Press, Oxford, pp 253–275
Rodday SM, Webber AN, Bingham SE, Biggins J (1995) Evidence that the Fx domain in photosystem I interacts with the subunit PsaC: site-directed changes in PsaB destabilize the subunit interaction inChlamydomonas reinhardtii. Biochemistry 34:6328–6334
Roffey R, Sayre R (1990) Isolation of site-specific chloroplastpsbA mutations inChlamydomonas having null phenotypes via cotransformation. Plant Physiol 93 (Suppl):22
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual (2nd edn). Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Southern EM (1975) Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98:503–517
Svab Z, Maliga P (1993) High efficiency plastid transformation in tobacco by selection for chimericaadA gene. Proc Natl Acad Sci USA 90:913–917
Takahashi Y, Goldschmidt-Clermont M, Soen S-Y, Franzen L-G, Rochaix J-D (1991) Directed chloroplast transformation inChlamydomonas reinhardtii: insertional inactiviation of thepsaC gene encoding the iron sulfur protein destabilizes photosystem I. EMBO J 10:2033–2040
Zerges W, Rochaix J-D (1994) The 5′leader of a chloroplast mRNA mediates the translational requirements for two nucleus-encoded functions inChlamydomonas reinhardtii. MCB 14:5268–5277
Author information
Authors and Affiliations
Additional information
Communicated by R. G. Herrmann
Rights and permissions
About this article
Cite this article
Fischer, N., Stampacchia, O., Redding, K. et al. Selectable marker recycling in the chloroplast. Molec. Gen. Genet. 251, 373–380 (1996). https://doi.org/10.1007/BF02172529
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02172529