Summary
The anion-exchange resin method for soil-phosphate extraction was investigated on 4 different soils under varying experimental conditions. The variables were: (a) the type of anion-exchange resin, (b) the anionic form of the resin, (c) the ratio between the amounts of resin, soil, and water, and (d) the time of shaking.
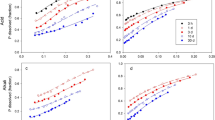
The amount of P extracted was dependent on the anionic form of the resin. For resins in the chloride form both the amount of P extracted psr soil unit and the pH of the soil suspension varied with the type of resin and the soil-water ratio.
Resins in the bicarbonate form stabilized the system, so that the amount of P extracted and the suspension pH were almost independent of the type of resin and the soil-water ratio.
The results indicated that the rate-determining step in the overall process of P transport from the soil phase through the water phase to the resin phase is the P desorption from the soil phase to the water phase, provided the resin is added in excess. The rate of this P desorption is dependent on the chemical composition of the water phase, which in turn is governed by the type of soil, the soil-water ratio, the time of shaking and the anionic form of the resin.
In a final experiment a resin was used in the chloride- and the bicarbonate form, respectively, for extraction of phosphate from 34 soils. The available P of these soils had been determined 15 years before in a pot experiment with ryegrass and by different laboratory methods2. The degree of correlation between the ryegrass P uptake and the P. determined by the laboratory methods decreased according to the following order: resin (bicarbonate form), resin (chloride form), 0.5M sodium bicarbonate, L value, E value, ammonium lactate solution, sodium zeolite, 0.01M calcium chloride, phosphate potential, and 0.1M sulphuric acid.
It is recommended that resins in the bicarbonate form should be used for both routine as well as more advanced analyses of the ability of soils to supply phosphate to plants. A final procedure for the analysis is given in the paper.
Similar content being viewed by others
References
Amer, F., Bouldin, D. R., Black, C. A. and Duke, F. R., Characterization of soil phosphorus by anion exchange resin adsorption and32P-equilibration. Plant and Soil6, 391–408 (1955).
Andersen, A. J. and Mogensen, T., A comparison of various laboratory methods for determining the phosphate condition in soils. Acta Agric. Scand.12, 315–323 (1962).
Aslyng, H. C., The lime and phosphate potentials of soils; the solubility and availability of phosphates. Yearb. Roy. Vet. Agric. Coll., Copenhagen, 1–50 (1954).
Bache, B. W. and Rogers, N. E., Soil phosphate values in relation to phosphate supply to plants from some Nigerian soils. J. Agric. Sci.74, 383–390 (1970).
Bergseth, H., Studien über Ionenaustausch. Thesis. Norges Landbrukshøgskole, Institut for Jordbunnslære, Vollebekk, pp. 240 (1969).
Bondorff, K. A., Studier over jordens fosforsyreindhold. V. En ny fremgangsmade ved undersøgelsen af jordens fosforsyreindhold. Tidsskr. Planteavl53, 336–342 (1950).
Cooke, I. J., and Hislop, J., Use of anion-exchange resin for the assessment of available soil phosphate. Soil Sci.96, 308–312 (1963).
Egnér, H., Riehm, H. and Domingo, W. R., Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nährstoffzustandes der Böden. II. Chemische Extraktionsmethoden zur Phosphor- und Kaliunibestimmung. Kungl. Landbrukshögsk. Ann.26, 199–215 (1960).
Elrashidi, M. A., Van Diest, A. and El-Damaty, A. H., Phosphorus determination in highly calcareous soils by the use of an anion exchange resin. Plant and Soil42, 273–286 (1975).
Hislop, J. and Cooke, I. J., Anion exchange resin as a means of assessing soil phosphate status: A laboratory technique. Soil Sci.105, 8–11 (1968).
Larsen, S., The use of32P in studies on the uptake of phosphorus by plants. Plant and Soil4, 1–10 (1952).
Metzger, W. H., The effect of growing plants on solubility of soil nutrients. Soil Sci.25, 273–280 (1928).
Moser, U. S., Sutherland, W. H. and Black, C. A., Evaluation of laboratory indexes of absorption of soil phosphorus by plants. I. Plant and Soil10, 356–374 (1959).
Murphy, J. and Riley, J. P., A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta27, 31–36 (1962).
Møller, J. and Mogensen, T., Use of an ion-exchanger for determining available phosphorus in soils. Soil Sci.76, 297–306 (1953).
Nagarajah, S., Posner, A. M. and Quirk, J. P., Desorption of phosphate from kaolinite by citrate and bicarbonate. Soil Sci. Soc. Am. Proc.32, 507–510 (1968).
Nye, P. H., Processes in the root environment. J. Soil Sci.19, 205–215 (1968).
Olsen, S. R., Cole, C. V., Watanabe, F. S. and Dean, L. A., Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U.S. Dept. Agr. Circ.939 (1954).
Riley, D. and Barber, S. A., Bicarbonate accumulation and pH changes at the soybean (Glycine max. (L) Merr.) root-soil interface. Soil Sci. Soc. Am. Proc.33, 905–908 (1969).
Riley, D. and Barber, S. A., Effect of ammonium and nitrate fertilization on phosphorus uptake as related to root-induced pH changes at the root-soil interface. Soil Sci. Soc. Am. Proc.35, 301–306 (1971).
Russell, R. S., Russell, E. W. and Marais, P. G., Factors affecting the ability of plants to absorb phosphate from soils. I. The relationship between labile phosphate and absorption. J. Soil Sci.8, 248–267 (1957).
Sibbesen, E., A simple ion-exchange resin procedure for extracting plant-available elements from soil. Plant and Soil46, 665–669 (1977).
Vaidyanathan, L. V. and Talibudeen, O., Rate processes in the desorption of phosphate from soils by ion exchange resins. J. Soil Sci.21, 173–183 (1970).
Walmsley, D. and Cornforth, I. S., Methods of measuring available nutrients in West Indian soils. II. Phosphorus. Plant and Soil39, 93–100 (1973).
Zunino, H., Aguilera, M. and Peirano, P., A modified resin exchange method for measurement of available phosphate in soils derived from volcanic ash. Soil Sci.114, 404–405 (1972).
Özbek, N. and Sadiq, M., A modified anion exchange resin equilibration method for the determination of available phosphorus in soils. Yearb. Fac. Agr. Univ. Ankara7, 95–106 (1967).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sibbesen, E. An investigation of the anion-exchange resin method for soil phosphate extraction. Plant Soil 50, 305–321 (1978). https://doi.org/10.1007/BF02107180
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02107180