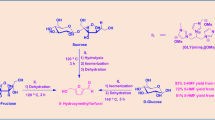
Abstract
Form ose reaction, the Ca(OH)2 catalyzed condensation of HCHO to a complex mixture of sugars, does not lose its autocatalytic nature at 98°C. The induction period is 15 sec, and then, at 18% total HCHO conversion, there was a period of approximately 3 sec duration in which the reaction had produced C6 sugars at 84.3 wt.% selectivity. Glucose amounted to 90% of those sugars, and no branched sugars were detected. Conversion was complete within 3 min. The simple nature of the product was lost at lower temperatures or higher conversions.
Abstract
Реакция формозы—конденсация HCHO, катализированная Ca(OH)2, до сложной смеси сахаров не теряет своего автокаталитического характера при 98°C. Индукционный период составляет 15 секунд, и затем при 38%-ой общей конверсии HCHO наблюдался период продолжительностью приблизительно в 3 секунды, когда реакция приводит к образованию сахаров C6 с селективностью 84,3 вес. %. Среди них глюкоза достигает 90%, а разветвленные сахара не удалось детектировать. Конверсия была завершена в течение 3-ех минут. При более низких температурах и повышенных конверсиях природа продукта усложняется.
Similar content being viewed by others
References
A. Butlerow: Ann.,120, 9 (1861).
T. Mizuno and A. H. Weiss, in “Advances in Carbohydrate Chemistry and Biochemistry” (R. S. Tipson and D. Horton, Eds.), Vol. 29, p. 173. Acacemic Press, New York 1974.
A. H. Weiss, J. A. Shapira: AIChE Symposium Series,67, 108, 137 (1971).
A. H. Weiss, R. La Pierre, J. A. Shapira: J. Catal.,16, 332 (1970).
A. H. Weiss, J. A. Shapira: Hydr. Proc.,49, 119–126 (1970).
A. H. Weiss, H. Tambawala: J. Catal.,26, 388 (1972).
R. D. Partridge, A. H. Weiss, D. Todd: J. Carbohydr. Res.,24, 28 (1972).
G. C. Akerlof: J. Spacecraft,1, 303 (1964).
J. Shapira: Cereal Science Today,13, No. 2 (1968).
A. H. Weiss, R. D. Partridge, H. Tambawala, J. A. Shapira, “Polyols from Formaldehyde”, Dechema-Monographien Nos. 1264–1291, Vol. 68, pp. 239–264. Verlag Chemie, Weinheim/Bergstrasse 1971.
A. H. Weiss, H. Tambawala: J. Chromatogr. Sci.,10, 120 (1972).
C. C. Sweeley, R. Bentley, M. Makita, W. W. Wells: J. Amer. Chem. Soc.,85, 2497 (1963).
T. Cayle, F. Viebrock, J. Schiaffino: Cereal Chemistry,48, 154 (1968).
A. H. Weiss, V. A. Seleznev, and R. D. Partridge, “Simultaneously Catalyzed Reactions of Formaldehyde in Alkaline Systems”, presented at Organic Reactions Catal. Soc., 6th Conf. Catal. Organic Systems, Boston, Mass., May 11, 1976. Proceedings in press.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Likholobov, V.A., Weiss, A.H. & Sakharov, M.M. The use of temperature to simplify formose sugar composition. React Kinet Catal Lett 8, 155–166 (1978). https://doi.org/10.1007/BF02061300
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02061300