Summary
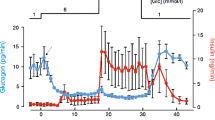
The control of K+ channels in the insulin-secreting cell line RINm5F has been investigated by patch-clamp singlechannel current recording experiments. The unitary current events recorded from cell-attached patches are due to large and small inwardly rectifying ATP-sensitive K+ channels with conductance properties similar to the two channels previously identified in primary cultured rat islet cells (Findlay, I., Dunne, M.J., & Petersen, O. H.J. Membrane Biol. 88:165–172, 1985). Cell permeabilization through brief exposure to 10 μm digitonin or 0.05% saponin (outside the isolated membrane patch area) results in a dramatic increase in current through the cell-attached patch due to opening of many large and small K+-selective channels. These channels are inhibited in a dose-dependent manner by ATP applied to the bath (near-complete inhibition by 5mm ATP). During prolonged ATP exposure (1–5 min) the initial inhibition is followed by partial recovery of channel activity, although further activation does occur when ATP is subsequently removed. From the maximal number of coincident channel openings in the permeabilized cells (in the absence of ATP), it is estimated that there are on average 12 large ATP-sensitive K+ channels per membrane patch, but in the intact cells less than 5% of the membrane patches exhibited three or more coincident K+ channel openings, indicating the degree to which the channels are inhibited in the resting condition by endogenous ATP. Stimulation of RINm5F cells to secrete insulin was carried out by challenging intact cells with 10mm d-glyceraldehyde.d-glyceraldehyde induced depolarization of the membrane from about −70 to −20 mV and evoked a marked reduction in the open-state probability of both the large and small ATP-sensitive channels.d-glyceraldehyde also induced action potentials in a number of cases. All effects of stimulation were largely transient, lasting about 100 sec. The two ATP-sensitive K+ channels are probably responsible for the resting potential and play a crucial role in coupling metabolism to membrane depolarization.
Similar content being viewed by others
References
Ashcroft, F.M., Ashcroft, S.J.H., Harrison, D.E. 1985. The glucose-sensitive potassium channel in rat pancreatic beta-cells is inhibited by intracellular ATP.J. Physiol. (London) 369:101P
Ashcroft, F.M., Harrison, D.E., Ashcroft, S.J.H. 1984. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells.Nature (London) 312:446–448
Cook, D.L., Hales, C.N. 1984. Intracellular ATP directly blocks K+ channels in pancreatic B-cells.Nature (London) 311:271–273
Cook, D.L., Ikeuchi, M., Fujimoto, W.Y. 1984. Lowering of pH i inhibits Ca2+-activated K+ channels in pancreatic B-cells.Nature (London) 311:269–271
Dean, P.M., Matthews, E.K. 1968. Electrical activity in pancreatic islet cells.Nature (London) 219:389–390
Dean, P.M., Matthews, E.K. 1970. Glucose-induced electrical activity in pancreatic islet cells.J. Physiol. (London) 210:255–264
Dean, P.M., Matthews, E.K., Sakamoto, Y. 1975. Pancreatic islet cells: Effects of monosaccharides, glycolytic intermediates and metabolic inhibitors on membrane potential and electrical activity.J. Physiol. (London) 246:459–478
Findlay, I., Dunne, M.J. 1985. Voltage-activated Ca2+ currents in insulin-secreting cells.FEBS Lett. 189:281–285
Findlay, I., Dunne, M.J., Petersen, O.H., 1985a. High-conductance K+ channel in pancreatic islet cells can be activated and inactivated by internal calcium.J. Membrane Biol. 83:169–175
Findlay, I., Dunne, M.J., Petersen, O.H. 1985b. ATP-sensitive inward rectifier and voltage-and calcium-activated K+ channels in cultured pancreatic islet cells.J. Membrane Biol. 88:165–172
Findlay, I., Dunne, M.J., Ullrich, S., Wollheim, C.B., Petersen, O.H. 1985c. Quinine inhibits Ca2+-independent K+ channels whereas tetraethylammonium inhibits Ca2+-activated K+ channels in insulin-secreting cells.FEBS Lett. 185:4–8
Halban, P.A., Praz, G.A., Wollheim, C.B. 1983. Abnormal glucose metabolism accompanies failure of glucose to stimulate insulin release from a pancreatic cell line (RINm5F)Biochem. J. 212:439–443
Hamill, O.P., Marty, A., Neher, E., Sakmann, B., Sigworth, F.J. 1981. Improved patch-clamp techniques for high resolution current recordings from cells and cell-free membrane patches.Pfluegers Arch. 391:85–100
Henquin, J.C., Meissner, H.P. 1984. Significance of ionic fluxes and changes in membrane potential for stimulus-secretion coupling in B-cells.Experientia 40:1043–1052
Kakei, M., Noma, A., Shibasaki, T. 1985. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells.J. Physiol. (London) 363:441–462
Latorre, R., Miller, C. 1983. Conduction and selectivity in potassium channels.J. Membrane Biol. 71:11–30
Maruyama, Y., Petersen, O.H., Flanagan, P., Pearson, G.T. 1983. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells.Nature (London) 305:228–232
Petersen, O.H., Maruyama, Y. 1984. Calcium-activated potassium channels and their role in secretion.Nature (London) 307:693–696
Praz, G.A., Halban, P.A., Wollheim, C.B., Blondel, B., Strauss, A.J., Renold, A.E. 1983. Regulation of immunoreactive-insulin release from a rat cell line (RINm5F).Biochem. J. 210:345–352
Rorsman, P., Trube, G. 1985. Glucose-dependent K+ channels in pancreatic B-cells are regulated by intracellular ATP.Pfluegers Arch. 405:305–309
Sehlin, J., Täljedal, I.-B. 1975. Glucose-induced decrease in Rb+ permeability in pancreatic beta cells.Nature (London) 253:635–636
Wollheim, C.B., Pozzan, T. 1984. Correlation between cytosolic free Ca2+ and insulin release in an insulin secreting cell line.J. Biol. Chem. 259:2262–2267
Wollheim, C.B., Ullrich, S., Pozzan, T. 1984. Glyceraldehyde but not cyclic AMP-stimulated insulin release is preceded by a rise in cytosolic free Ca2+.FEBS Lett. 177:17–22
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dunne, M.J., Findlay, I., Petersen, O.H. et al. ATP-sensitive K+ channels in an insulin-secreting cell line are inhibited byd-glyceraldehyde and activated by membrane permeabilization. J. Membrain Biol. 93, 271–279 (1986). https://doi.org/10.1007/BF01871181
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01871181