Summary
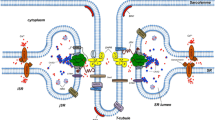
Isolated triadic proteins were employed to investigate the molecular architecture of the triad junction in skeletal muscle. Immunoaffinity-purified junctional foot protein (JFP), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), aldolase and partially purified dihydropyridine (DHP) receptor were employed to probe protein-protein interactions using affinity chromatography, protein overlay and crosslinking techniques. The JFP, an integral protein of the sarcoplasmic reticulum (SR) preferentially binds to GAPDH and aldolase, peripheral proteins of the transverse (T)-tubule. No direct binding of JFP to the DHP receptor was detected. The interactions of JFP with GAPDH and aldolase appear to be specific since other glycolytic enzymes associated with membranes do not bind to the JFP. The DHP receptor, an integral protein of the T-tubule, also binds GAPDH and aldolase. A ternary complex between the JFP and the DHP receptor can be formed in the presence of GAPDH. In addition, the DHP receptor binds to a previously undetectedM r 95 K protein which is distinct from the SR Ca2+ pump and phosphorylaseb. TheM r 95 K protein is an integral protein of the junctional domain of the SR terminal cisternae. It is also present in the newly identified “strong triads” (accompanying paper). From these findings, we propose a new model for the triad junction.
Similar content being viewed by others
References
Arnold, H., Pette, D. 1968. Binding of glycolytic enzymes to structural proteins of the muscle.Eur. J. Biochem. 6:163–171
Arnold, H., Pette, D. 1970. Binding of aldolase and triosephosphate dehydrogenase to F-actin and modification of catalytic properties of aldolase.Eur. J. Biochem. 15:360–368
Block, B.A., Imagawa, T., Campbell, K.P., Franzini-Armstrong, C. 1988. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle.J. Cell. Biol. 107:2587–2600
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principles of protein dye binding.Anal. Biochem. 72:248–254
Brandt, N.R., Caswell, A.H., Brunschwig, J.-P. 1980. ATP-energized Ca2+ pump in isolated transverse tubules of skeletal muscle.J. Biol. Chem. 255:6290–6298
Brunschwig, J.-P., Brandt, N., Caswell, A.H., Lukeman, D.S. 1982. Ultrastructural observations of isolated intact and fragmented junctions of skeletal muscle by use of tunnic acid mordanting.J. Cell Biol. 93:533–542
Cadwell, J.J.S., Caswell, A.H. 1982. Identification of a constituent of the junctional feet linking terminal cisternae to transverse tubules in skeletal muscle.J. Cell Biol. 93:543–550
Caswell, A.H., Brandt, N.R. 1981. Correlations of Ca2+ release from terminal cisternae with integrity of triad junction.In: The Mechanism of Gated Calcium Transport Across Biological Membranes. T. Ohnishi and M. Endo, editors. pp. 219–226. Academic, New York
Caswell, A.H., Corbett, A.M. 1985. Interaction of glyceraldehyde-3-phosphate dehydrogenase with isolated muscle subfractions of skeletal muscle.J. Biol. Chem. 260:6892–6898
Caswell, A.H., Lau, Y.H., Brunschwig, J.-P. 1976. Ouabain-binding vesicles from skeletal muscle.Arch. Biochem. Biophys 176:417–430
Catterall, W.A., Seagar, M.J., Takahashi, M. 1988. Molecular properties of dihydropyridine-sensitive calcium channels in skeletal muscle.J. Biol. Chem. 263:3535–3538
Chadwick, C.C., Inui, M., Fleischer, S. 1988. Identification and purification of a transverse tubule coupling protein which binds to the ryanodine receptor of terminal cisternae at the triad junction in skeletal muscle.J. Biol. Chem. 236:10872–10877
Corbett, A.M., Caswell, A.H., Brandt, N.R., Brunschwig, J.-P. 1985. Determinants of triad junction reformation: Identification and isolation of an endogenous promotor for junction reformation in muscle.J. Membrane Biol. 86:267–276
Curtis, B.M., Catterall, W.A. 1984. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules.Biochemistry 23:2113–2118
Curtis, B.M., Catterall, W.A. 1985. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase.Proc. Natl. Acad. Sci. USA 82:2528–2532
Eisenberg, B.R., Gilai, A. 1979. Structural changes in single muscle fibres after stimulation at a low frequency.J. Gen. Physiol. 74:1–16
Endo, M., Tanaka, M., Ogawa, Y. 1970. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres.Nature (London) 228:34–36
Ford, L.E., Podolsky, R.J. 1970. Regenerative calcium release within muscle cells.Science 167:58–59
Hossenlopp, P., Seurin, D., Segovia-Quinson, B., Hardouin, S., Binouix, M. 1986. Analysis of in serum insulin-like growth factor binding proteins using Western blotting: Use of the method for titration of the binding proteins and competitive binding studies.Anal. Biochem. 154:138–143
Hymel, L., Inui, M., Fleischer, S., Schindler, H.G. 1987. Purified skeletal muscle ryanodine receptor forms Ca2+-activated multisubunit Ca2+ channels in planar bilayers.Proc. Natl. Acad. Sci. USA 85:411–445
Imagawa, T., Smith, J.S., Coronado, R., Campbell, K.P. 1987. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel.J. Biol. Chem. 262:636–643
Inui, M., Saito, A., Fleischer, S. 1987. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle.J. Biol. Chem. 262:1740–1747
Kawamoto, R.M., Brunschwig, J.-P., Caswell, A.H. 1988. Localization by immunoelectron microscopy of spanning protein of triad junction in terminal cisternae/triad vesicles.J. Muscle Res. Cell Motil. 9:334–343
Kawamoto, R.M., Brunschwig, J.-P., Kim, K.C., Caswell, A.H. 1986. Isolation, characterization and localization of the spanning protein from skeletal muscle triads.J. Cell Biol. 103:1405–1414
Kim, K.C., Caswell, A.H., Brunschwig, J.-P., Brandt, N.R. 1990. Identification of a new subpopulation of triad junctions isolated from skeletal muscle: Morphological correlations with intact muscle.J. Membrane Biol. 113:221–235
Koppitz, B., Vogel, F., Mayr, G.W. 1986. Mammalian aldolases are isomer selective high affinity inositol polyphosphate binders.Eur. J. Biochem. 1:421–433
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage-T4.Nature (London) 277:680–685
Lai, F.A., Erickson, H.P., Rousseau, E., Liu, Q.-Y., Meissner, G. 1988. Purification and reconstitution of the calcium release channel from skeletal muscle.Nature (London) 231:315–319
Lau, Y.H., Caswell, A.H., Brunschwig, J.-P. 1977. Isolation of transverse tubules by fractionation of triad junctions of skeletal muscle.J. Biol. Chem. 252:5565–5574
Pessah, I.N., Franzini, A.O., Scales, D.J., Waterhouse, A.L., Cassida, J.E. 1986. Calcium ryanodine receptor complex.J. Biol. Chem. 261:8643–8648
Pierce, G.N., Phillipson, K.D. 1985. Binding of glycolytic enzymes to cardiac sarcolemma and sarcoplasmic reticulum membranes.J. Biol. Chem. 260:6862–6870
Rios, E., Brum, G. 1987. Involvement of dihydropyridine receptors in excitation-contraction coupling in skeletal muscle.Nature (London) 325:717–720
Schneider, M.F., Chandler, W.K. 1973. Voltage dependent charge movement in skeletal muscle: A possible step in excitation-contraction coupling.Nature (London) 242:244–246
Seiler, S., Wegener, A.D., Whang, D.D., Hathaway, D.R., Jones, L.R. 1983. High molecular weight proteins in cardiac and skeletal muscle junctional sarcoplasmic reticulum vesicles bind calmodulin, are phosphorylated, and are degraded by Ca2+-activated protease.J. Biol. Chem. 258:8550–8557
Sock, J., Rohringer, R. 1988. Activity staining of blotted enzymes by reaction coupling with transfer membrane-immobilized auxillary enzymes.Anal. Biochem. 171:310–319
Talvenheimo, J.A., Worley, J.F., III, Nelson, M.T. 1987. Heterogeneity of calcium channels from a purified dihydropyridine receptor preparation.Biophys. J. 52:891–899
Tanabe, T., Takeshima, H., Mikami, A., Flockenzi, V., Takahashi, H., Kangawa, K., Kojima, M., Matsuo, H., Hirose, T., Numa, S. 1987. Primary structure of the receptor for calcium channel blockers from skeletal muscle.Nature (London) 328:313–318
Thieleczek, R., Mayr, G.W., Brandt, N.R. 1989. Inositol polyphosphate-mediated repartitioning of aldolase in skeletal muscle triads and myofibrils.J. Biol. Chem. 264:7349–7356
Tsai, I.-H., Murphy, S.N.P., Steck, T.L. 1982. Effect of red cell membrane binding on the catalytic activity of glyceraldehyde-3-phosphate dehydrogenase.J. Biol. Chem. 257:1438–1442
Vergara, J., Tsien, R.Y., Delay, M. 1985. Inoistol 1,4,5-trisphosphate: A possible chemical link in excitation-contraction coupling in muscle.Proc. Natl. Acad. Sci. USA 82:6352–6356
Vilven, J., Coronado, R. 1988. Opening of dihydropyridine calcium channels in skeletal muscle membranes by inositol trisphosphate.Nature (London) 336:567–589
Volpe, P., Salviati, G., DiVirgilio, F., Pozzan, T. 1985. Inositol 1,4,5 trisphosphate induces calcium release from sarcoplasmic reticulum of skeletal muscle.Nature (London) 3:347–349
Zorzato, F., Volpe, P. 1988. Calcium binding proteins of junctional sarcoplasmic reticulum: Detection by45Ca overlay.Arch. Biochem. Biophys. 261:324–329
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brandt, N.R., Caswell, A.H., Wen, SR. et al. Molecular interactions of the junctional foot protein and dihydropyridine receptor in skeletal muscle triads. J. Membrain Biol. 113, 237–251 (1990). https://doi.org/10.1007/BF01870075
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01870075