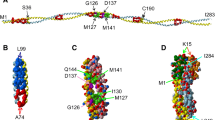
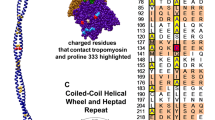
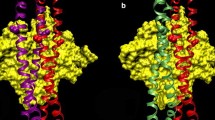
Summary
Steric blocking of actin-myosin interaction by tropomyosin has been a working hypothesis in the study of the regulation of skeletal muscle contraction, yet the simple movement of actin-associated tropomyosin from a myosin-blocking position (relaxation) to a nonblocking position (contraction) cannot adequately account for all of the biophysical and biochemical observations which have been made to date. Ambiguous assignment of tropomyosin positions on actin during contraction, due in part to the limited resolution of reconstruction techniques, may also hint at a real lack of clearcut ‘on’ and ‘off’ positioning of tropomyosin and tropomyosin-troponin complex. Recent biochemical evidence suggests processes relatively independent of tropomyosin-troponin may have a governing effect on contraction, involving kinetic constraints on actin-myosin interaction influenced by the binding of ATP and the intermediates of ATP hydrolysis. Based on our current understanding put forth in this review, it is clear that regulatory interactions in muscle contraction do not consist solely of steric effects but involve kinetic factors as well. Where the latter are being defined in systems reconstituted from purified proteins and their fragments, the steric components of regulation are most clearly observed in studies of structurally more intact physiologic systems (e.g. intact or skinned whole muscle fibres). The fine detail of the processes and their interplay remains an intriguing question. Likewise, the precise physical relationship of myosin with actin in the crossbridge cycle continues to elude definition. Refinement of several methodologies (X-ray crystallography, three-dimensional reconstruction, time-resolved X-ray diffraction) will increase the potential for detailing the molecular basis of the regulation of muscle contraction.
Similar content being viewed by others
References
Adrian, M., Dubochet, J. Lepault, J. &McDowall, A. W. (1984) Cryoelectron microscopy of viruses.Nature, Lond. 308, 32–6.
Bálint, M., Wolf, I., Tarcsafalvi, A., Gergely, J. &Sréter, F. A. (1978) Location of SH-1 and SH-2 in the heavy chain segment of heavy meromyosin.Archs Biochem. Biophys. 190, 793–9.
Berzofsky, J. A., Buckenmyer, G. K., Hicks, G., Gurd, F. R. N., Feldmann, R. J. &Minna, J. (1982) Topographic antigenic determinants recognized by monoclonal antibodies to sperm whale myoglobin.J. biol. Chem. 257, 3189–98.
Brandt, P. W., Cox, R. N. &Kawai, M. (1980) Can the binding of Ca2+ to two regulatory sites on troponin C determine the steep pCa/tension relationship of skeletal muscle?Proc. natn. Acad. Sci. U.S.A. 77, 4717–20.
Brandt, P. W., Reuben, J. P. &Grundfest, H. (1972) Regulation of tension in the skinned crayfish muscle fiber. II. Role of calcium.J. gen. Physiol. 59, 305–17.
Bremel, R. D. &Weber, A. (1972) Cooperation within actin filament in vertebrate skeletal muscle.Nature, New Biol. 238, 97–101.
Brenner, B., Schoenberg, M., Chalovich, J. M., Green, L. E. &Eisenberg, E. (1982) Evidence for cross-bridge attachment in relaxed muscle at low ionic strength.Proc. natn. Acad. Sci. U.S.A. 79, 7288–91.
Brenner, B., Yu, L. C. &Podolsky, R. J. (1984) X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths.Biophys. J. 46, 299–306.
Burke, M. &Reisler, E. (1977) Effect of nucleotide binding on the proximity of the essential sulfhydryl groups of myosin. Chemical probing of movement of residues during conformational transitions.Biochemistry 16, 5559–63.
Carnegie, P. R., Dowse, C. A. &Linthicum, D. S. (1983) Antigenic determinant recognized by a monoclonal antibody to human myelin basic protein.J. Neuroimmun. 5, 125–34.
Chalovich, J. M., Chock, P. B. &Eisenberg, E. (1981) Mechanism of action of troponin-tropomyosin. Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin.J. biol. Chem. 256, 575–8.
Chalovich, J. M. &Eisenberg, E. (1982) Inhibition of actomyosin ATPase by troponin-tropomyosin without blocking the binding of myosin to actin.J. biol. Chem. 257, 2432–7.
Chalovich, J. M. &Eisenberg, E. (1984) The effect of troponin-tropomyosin on the binding of skeletal muscle HMM to actin in the presence of ATP.Biophys. J. 45, 221a.
Chalovich, J. M., Greene, L. E. &Eisenberg, E. (1983) Crosslinked myosin subfragment 1: A stable analogue of the subfragment-1· ATP complex.Proc. natn. Acad. Sci. U.S.A. 80, 4909–13.
Cooke, R. (1981) Stress does not alter the conformation of a domain of the myosin cross-bridge in rigor muscle fibres.Nature, Lond. 294, 570–1.
Cooke, R., Crowder, M. S. &Thomas, D. D. (1982) Orientation of spin labels attached to cross-bridges in contracting muscle fibres.Nature, Lond. 300, 776–8.
Donaldson, S. K. B. &Kerrick, W. G. L. (1975) Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers.J. gen. Physiol. 66, 427–44.
East, I. J., Hurrell, J. G. R., Todd, P. E. E. &Leach, S. J. (1982) Antigenic specificity of monoclonal antibodies to human myoglobin.J. biol. Chem. 257, 3199–202.
Eaton, B. L. (1976) Tropomyosin binding to F-actin induced by myosin heads.Science, N. Y. 192, 1337–9.
Eaton, B. L., Kominz, D. R. &Eisenberg, E. (1975) Correlation between the inhibition of the acto-heavy meromyosin ATPase and the binding of tropomyosin to F-actin: Effects of Mg2+, KCl, troponin I and troponin C.Biochemistry 14, 2718–24.
Ebashi, S. (1980) Regulation of muscle contraction.Proc. R. Soc. Ser. B. 207, 259–86.
Ebashi, S. &Endo, M. (1968) Calcium ion and muscle contraction.Prog. Biophys. Molec. Biol. 18, 123–83.
Egelman, E. H. (1985) The structure of F-actin.J. Musc. Res. Cell Motility 6, 129–51.
Egelman, E. H. &DeRosier, J. J. (1983) Structural studies of F-actin. InActin: Structure and Function in Muscle and Non-Muscle Cells (edited byDos Remedios, C. G. &Barden, J. A.), pp. 17–24. Sydney, Australia: Academic Press, Inc.
El-Saleh, S. C. &Potter, J. D. (1985) Calcium-insensitive binding of heavy meromyosin to regulated actin. Interaction under physiological ionic strength.J. biol. Chem. 260, 14775–9.
El-Saleh, S. C., Thieret, R., Johnson, P. &Potter, J. D. (1984) Modification of Lys-237 on actin by 2,4-pentanedione. Alteration of the interaction of actin with tropomyosin.J. biol. Chem. 259, 11014–21.
Fabiato, A. &Fabiato, F. (1978) Myofilament-generated tension oscillations during partial calcium activation and activation dependence of the sarcomere length-tension relation of skinned cardiac cells.J. gen. Physiol. 72, 667–99.
Fajer, P., Fajer, E., Svensson, E., Brunsvold, N., Wendt, C. &Thomas, D. (1985) EPR studies of muscle contraction at low ionic strength (IS).Biophys. J. 47, 380a.
Filo, R. S., Bohr, D. F. &Rüegg, J. C. (1965) Glycerinated skeletal and smooth muscle: Calcium and magnesium dependence.Science, N. Y. 147, 1581–3.
Fuchs, F. (1977) Cooperative interactions between calcium-binding sites on glycerinated muscle fibers. The influence of cross-bridge attachment.Biochim. biophys. Acta 462, 314–22.
Gilbert, H. F., III &O'Leary, M. H. (1975) Modification of arginine and lysine in proteins with 2,4-pentanedione.Biochemistry 14, 5194–9.
Greaser, M. L. &Gergely, L. (1973) Purification and properties of the components from troponin.J. biol. Chem. 248, 2125–33.
Greene, L. (1982) The effect of nucleotide on the binding of myosin subfragment 1 to regulated actin.J. biol. Chem. 257, 13993–9.
Greene, L. E. &Eisenberg, E. (1980) Cooperative binding of myosin subfragment-1 to the actin-troponintropomyosin complex.Proc. natn. Acad. Sci. U.S.A. 77, 2616–20.
Greene, L. E., Sellers, J. R., Eisenberg, E. &Adelstein, R. S. (1983) Binding of gizzard smooth muscle myosin subfragment 1 to actin in the presence and absence of adenosine 5′-triphosphate.Biochemistry 22, 530–5.
Harrington, W. F. (1971) A mechanochemical mechanism for muscle contraction.Proc. natn. Acad. Sci. U.S.A. 68, 685–9.
Harrington, W. F. (1979) On the origin of the contractile force in skeletal muscle.Proc. natn. Acad. Sci. U.S.A. 76, 5066–70.
Harrington, W. F. &Rodgers, M. E. (1984) Myosin.A. Rev. Biochem. 53, 35–73.
Haselgrove, J. C. (1972) X-ray evidence for a conformational change in the actin containing filaments of vertebrate striated muscle.Cold Spring Harb. Symp. quant. Biol. 37, 341–52.
Haselgrove, J. C. (1975) X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle.J. molec. Biol. 92, 113–43.
Haselgrove, J. C. (1980) A model of myosin crossbridge structure consistent with the low-angle X-ray diffraction pattern from vertebrate muscle.J. Musc. Res. Cell Motility 2, 177–91.
Hellam, D. C. &Podolsky, R. J. (1969) Force measurements in skinned muscle fibers.J. Physiol., Lond. 200, 807–19.
Hill, D. K. (1968) Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation.J. Physiol., Lond. 199, 637–84.
Hill, T. L. (1952) Some statistical mechanical models of elastic polyelectrolytes and proteins.J. Chem. Phys. 70, 1259–73.
Hill, T. L. (1983) Two elementary models for the regulation of skeletal muscle contraction by calcium.Biophys. J. 44, 383–96.
Hill, T. L., Eisenberg, E. &Greene, L. (1980) Theoretical model for the cooperative equilibrium binding of myosin subfragment 1 to the actin-troponin-tropomyosin complex.Proc. natn. Acad. Sci. U.S.A. 77, 3186–90.
Hill, T. L., Eisenberg, E. &Greene, L. E. (1983) Alternate model for the cooperative equilibrium binding of myosin subfragment-1-nucleotide complex to actin-troponin-tropomyosin.Proc. natn. Acad. Sci. U.S.A. 80, 60–4.
Hitchcock, S. E. (1975) Regulation of muscle contraction: Binding of troponin and its components to actin and tropomyosin.Eur. J. Biochem. 52, 255–63.
Hitchcock, S. E., Zimmerman, C. J. &Smalley, C. (1981) Study of the structure of troponin-T by measuring the relative reactivities of lysines with acetic anhydride.J. molec. Biol. 147, 125–51.
Horwitz, J., Bullard, B. &Mercola, D. (1979) Interaction of troponin subunits. The interaction between the inhibitory and tropomyosin-binding subunits.J. biol. Chem. 254, 350–55.
Hozumi, T. &Muhlrad, A. (1981) Reactive lysyl of myosin subfragment 1: Location on the 27 K fragment and labeling properties.Biochemistry 20, 2945–50.
Huxley, A. F. &Simmons, R. M. (1971) Proposed mechanism of force generation in striated muscle.Nature, Lond. 233, 533–8.
Huxley, H. E. (1969) The mechanism of muscular contraction.Science, N. Y. 164, 1356–66.
Huxley, H. E. (1972) Structural changes in the actin and myosin containing filaments during contraction.Cold Spring Harb. Symp. quant. Biol. 37, 361–76.
Huxley, H. E. (1982) Molecular basis of contraction in cross-striated muscles and relevance to the motile mechanisms in other cells. InMuscle and Nonmuscle Matility (edited byStracher, A.) pp. 1–104. New York: Academic Press, Inc.
Huxley, H. E. &Brown, W. (1967) The low-angle X-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor.J. molec. Biol. 30, 383–434.
Huxley, H. E., Faruqi, A. R., Bordas, J., Koch, M. H. J. &Mitch, J. R. (1980) The use of synchrotron radiation in time-resolved X-ray diffraction studies of myosin layer-line reflections during muscle contraction.Nature, Lond. 284, 140–3.
Huxley, H. E., Simmons, R. M., Faruqi, A. R., Kress, M., Bordas, J. &Koch, M. H. J. (1983) Changes in the X-ray reflections from contracting muscle during rapid mechanical transients and their structural implications.J. molec. Biol. 169, 469–506.
Julian, F. J. (1971) The effect of calcium on the force-velocity relation of briefly glycerinated frog muscle fibres.J. Physiol., Lond. 218, 117–45.
King, R. T. &Greene, L. E. (1985) Regulation of the adenosinetriphosphatase activity of cross-linked actinmyosin subfragment 1 by troponin-tropomyosin.Biochemistry 24, 7009–14.
Labbé, J.-P., Mornet, D., Roseau, G. &Kassab, R. (1982) Cross-linking of F-actin to skeletal myosin subfragment 1 with Bis(imido esters): Further evidence for the interaction of myosin-head heavy chain with an actin dimer.Biochemistry 21, 6897–902.
Leavis, P. C., Rosenfeld, S. S., Gergely, J., Grabarek, Z. &Drabikowski, W. (1978) Proteolytic fragments of troponin-C. Localization of high and low affinity Ca2+ binding sites and interactions with troponin I and troponin T.J. biol. Chem. 253, 5452–9.
Lehrer, S. S. &Morris, E. P. (1982) Dual effects of tropomyosin and troponin-tropomyosin on actomyosin subfragment 1 ATPase.J. biol. Chem. 257, 8073–80.
Lovell, S. J. &Winzor, D. J. (1977) Self-association of troponin.Biochem. J. 167, 131–6.
Lymn, R. W. (1975) Low angle X-ray diagrams from skeletal muscle: The effect of AMP-PNP, a non-hydrolyzed analogue of ATP.J. molec. Biol. 99, 567–82.
Lymn, R. W. &Taylor, E. W. (1971) Mechanism of adenosine triphosphate hydrolysis by actomyosin.Biochemistry 10, 4617–24.
McCubbin, W. D. &Kay, C. M. (1980) Calcium induced conformational changes in the troponin-tropomyosin complexes of skeletal and cardiac muscle and their roles in the regulation of contraction-relaxation.Acc. Chem. Res. 13, 185–92.
McLachlan, A. D. (1984) Structural implications of the myosin amino acid sequence.A. Rev. Biophys. Bioeng. 13, 167–89.
Mahmood, R. &Yount, R. G. (1984) Photochemical probes of the active site of myosin. Irradiation of trapped 3′-o-(4-benzoyl)benzoyladenosine 5′-triphosphate labels the 50-kilodalton heavy chain tryptic peptide.J. biol. Chem. 259, 12956–9.
Marston, S. &Tregear, R. T. (1974) Calcium binding and the activation of fibrillar insect flight muscle.Biochim. biophys. Acta 347, 311–18.
Mendelson, R. A. &Wilson, M. G. A. (1982) Three-dimensional disorder of dipolar probes in a helical array. Application to muscle cross-bridges.Biophys. J. 39, 221–7.
Miller, A. &Tregear, R. T. (1972) Structure of insect fibrillar flight muscle in the presence and absence of ATP.J. molec. Biol. 70, 85–104.
Monod, J., Wyman, J. &Changeux, J. (1965) On the nature of allosteric transitions: A plausible model.J. molec. Biol. 12, 88–118.
Mornet, D., Bertrand, R., Pantel, P., Audemard, E. &Kassab, R. (1981) Structure of the actin-myosin interface.Nature, Lond. 292, 301–6.
Mornet, D., Pantel, R., Bertrand, R., Audemard, E. &Kassab, R. (1980) Localization of the reactive trinitrophenylated lysyl residue of myosin ATPase site in the NH2-terminal (27K domain) of S1 heavy chain.FEBS Lett. 117, 183–8.
Murray, J. M., Knox, M. K., Trueblood, C. E. &Weber, A. (1980) Do tropomyosin and myosin compete for actin sites in the presence of calcium?FEBS Lett. 114, 169–73.
Murray, J. M., Knox, M. K., Trueblood, C. E. &Weber, A. (1982) Potentiated state of the tropomyosin actin filament and nucleotide-containing myosin subfragment 1.Biochemistry 21, 906–15.
Murray, J. M., Weber, A. &Knox, M. K. (1981) Myosin subfragment 1 binding to relaxed actin filaments and steric model of relaxation.Biochemistry 20, 641–9.
Nagashima, H. &Asakura, S. (1982) Studies on co-operative properties of tropomyosin-actin and tropomyosin-troponin-actin complexes by the use ofN-ethylmaleimide-treated and untreated species of myosin subfragment 1.J. molec. Biol. 155, 409–28.
Naylor, G. R. S. &Podolsky, R. J. (1981) X-ray diffraction of strained muscle fibers in rigor.Proc. natn. Acad. Sci. U.S.A. 78, 5559–63.
O'Brien, E. J., Counch, J., Johnson, G. R. P. &Morris, E. P. (1983) Structure of actin and thin filament. InActin: Its Structure and Function in Muscle and Non-Muscle Cells (edited byDos Remedios, C. G. andBarden, J. A.), pp. 3–16. Sydney, Australia: Academic Press, Inc.
Parry, D. A. D. &Squire, J. M. (1973) The structural role of tropomyosin in muscle regulation: Analysis of the X-ray diffraction patterns from relaxed and contracting muscles.J. molec. Biol. 75, 33–55.
Perry, S. V. (1979) The regulation of contractile activity in muscle.Biochem. Soc. Trans. 7, 593–617.
Perry, S. V., Cole, H. A., Head, J. F. &Wilson, F. J. (1972) Localization and mode of action of the inhibitory protein component of the troponin complex.Cold Spring Harb. Symp. quant. Biol. 37, 251–62.
Pollard, T. D., Weeds, A. G. &Huxley, H. E. (1985) A rapid-freezing/stopped flow method to prepare the intermediates in the actin-myosin ATPase cycle for electron microscopy.Biophys. J. 47, 129a.
Potter, J. D. &Gergely, J. (1974) Troponin, tropomyosin and actin interactions in the Ca2+ regulation of muscle contraction.Biochemistry 13, 2697–703.
Potter, J. D. &Gergely, J. (1975) The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar adenosine triphosphatase.J. biol. Chem. 250, 4628–33.
Potter, J. D. &Johnson, J. D. (1982) Troponin. InCalcium and Cell Function, Vol. II (edited byCheung, Y.), pp. 145–73. New York: Academic Press, Inc.
Poulsen, F. R. &Lowy, J. (1983) Small-angle X-ray scattering from myosin heads in relaxed and rigor frog skeletal muscle.Nature, Lond. 303, 146–52.
Reedy, M. K. (1968) Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in the rigor cross-bridge lattice.J. molec. Biol. 31, 155–76.
Reedy, M. K., Holmes, K. C. &Tregear, R. T. (1965) Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle.Nature, Lond. 207, 1276–80.
Reisler, E., Burke, M. &Harrington, W. F. (1974) Cooperative role of two sulfhydryl groups in myosin adenosine triphosphatase.Biochemistry 13, 2014–22.
Reuben, I. P., Brandt, P. W., Berman, M. &Grundfest, H. (1971) Regulation of tension in the skinned crayfish muscle fiber. I. Contraction and relaxation in the absence of Ca (pCa > 9).J. gen. Physiol. 57, 385–407.
Rome, E. (1972) Relaxation of glycerinated muscle: Low-angle X-ray diffraction studies.J. molec. Biol. 65, 331–45.
Schoenberg, M., Brenner, B., Chalovich, J. M., Greene, L. E. &Eisenberg, E. (1984) Cross-bridge attachment in relaxed muscle. InContractile Mechanisms in Muscle (edited byPollack, G. H. andSugi, H.), pp. 269–84. New York: Plenum Publishing Corp.
Seidel, J. C., Chopek, M. &Gergely, J. (1970) Effect of nucleotides and pyrophosphate labels bound to S1 thiol groups of myosin.Biochemistry 9, 3265–72.
Smith-Gill, S. J., Lavoie, T. B. &Mainhart, C. R. (1984) Antigenic regions defined by monoclonal antibodies correspond to structural domains of avian lysozyme.J. Immun. 133, 384–93.
Smith-Gill, S. J., Wilson, A. C., Potter, M., Prager, E. M., Feldmann, R. J. &Mainhart, C. R. (1982) Mapping the antigenic epitope for a monoclonal antibody against lysozyme.J. Immun. 128, 314–22.
Squire, J. M. (1975) Muscle filament structure and muscle contraction.A. Rev. Biophys. Bioeng. 4, 137–63.
Squire, J. M. (1981) Thin filament structure and function. InThe Structural Basis of Muscular Contraction, pp. 157–218. New York: Plenum Press.
Stewart, M., Kensler, R. W. &Levine, R. J. C. (1981) Structure ofLimulus telson muscle thick filaments.J. molec. Biol. 153, 781–90.
Suck, D., Kabsch, W. &Mannherz, H. G. (1981) Three-dimensional structure of the complex of skeletal muscle actin and bovine pancreatic DNase I at 6 Å resolution.Proc. natn. Acad. Sci. U.S.A. 78, 4319–23.
Sutoh, K. (1982) Identification of myosin-binding sites on the actin sequence.Biochemistry 21, 3654–61.
Syska, H., Wilkinson, J. M., Grand, R. J. A. &Perry, S. V. (1976) The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit.Biochem. J. 153, 375–87.
Szilagyi, L., Bálint, M., Sréter, F. A. &Gergely, J. (1979) Photoaffinity labeling with an ATP analog of theN-terminal peptide of myosin.Biochem. Biophys. Res. Commun. 87, 936–45.
Tajima, Y., Kamiya, K. &Seto, T. (1983) X-ray structure analysis of thin filaments of a molluscan smooth muscle in the living relaxed state.Biophys. J. 43, 335–43.
Taylor, K. A. &Amos, L. A. (1981) A new model for the geometry of the binding of myosin crossbridges to muscle thin filaments.J. molec. Biol. 147, 297–324.
Taylor, K. A. &Amos, L. A. (1983) Structure of actin in reconstructed images of S-1 decorated filaments: A further comment. InActin: Structure and Function in Muscle and Non-Muscle Cells (edited byDos Remedios, C. G. andBarden, J. A.), pp. 25–6. Sydney, Australia: Academic Press, Inc.
Thomas, D. D. &Cooke, R. (1980) Orientation of spin-labeled myosin heads in glycerinated muscle fibers.Biophys. J. 32, 891–906.
Thomas, D. D., Ishiwata, S., Seidel, J. C. &Gergely, J. (1980) Submillisecond rotational dynamics of spinlabeled myosin heads in myofibrils.Biophys. J. 32, 873–89.
Todd, P. E. E., East, I. J. &Leach, S. J. (1982) The immunogenicity and antigenicity of proteins.TIBS 7, 212–6.
Vibert, P. &Craig, R. (1982) Three-dimensional reconstruction of thin filaments decorated with a Ca2+-regulated myosin.J. molec. Biol. 157, 299–319.
Wagner, P. D. (1984) Effect of skeletal muscle myosin light chain 2 on the Ca2+-sensitive interaction of myosin and heavy meromyosin with regulated actin.Biochemistry 23, 5950–6.
Wagner, P. D. &Giniger, E. (1981) Calcium-sensitive binding of heavy meromyosin to regulated actin in the presence of ATP.J. biol. Chem. 256, 12647–50.
Wagner, P. D. &Stone, D. B. (1983) Calcium-sensitive binding of heavy meromyosin to regulated actin requires light chain 2 and the head-tail junction.Biochemistry 22, 1334–42.
Wakabayashi, T., Huxley, H. E., Amos, L. A. &Klug, A. (1975) Three-dimensional image reconstruction of actin-tropomyosin complex and actin-tropomyosin-troponin T-troponin I complex.J. molec. Biol. 93, 477–97.
Wakabayashi, T. &Toyoshima, C. (1981) Three-dimensional image analysis of the complex of thin filaments and myosin molecules from skeletal muscle. II. The multi-domain structure of actin-myosin S1 complex.J. Biochem., Tokyo 90, 683–701.
Wakabayashi, T., Toyoshima, C. &Hosoi, M. (1983) Image analysis of actin-tropomyosin-myosin S-1. InActin: Structure and Function in Muscle and Non-Muscle Cells (edited byDos Remedios, C. G. andBarden, J. A.), pp. 27–34. Sydney, Australia: Academic Press, Inc.
Weeks, R. A. &Perry, S. V. (1977) A region of the troponin C molecule involved in interaction with troponin I.Biochem. Soc. Trans. 5, 1391–2.
Wells, J. A., Werber, M. M., Legg, J. I. &Yount, R. G. (1979a) Inactivation of myosin subfragment one by cobalt (II)/cobalt (III) phenanthroline complexes. 1. Incorporation of Co(III) byin situ oxidation of Co(II).Biochemistry 18, 4793–9.
Wells, J. A., Werber, M. M. &Yount, R. G. (1979b) Inactivation of myosin subfragment 1 by cobalt (II)/cobalt (III) phenanthroline complexes. 2. Cobalt chelation of two critical SH groups.Biochemistry 18, 4800–5.
Wells, J. A. &Yount, R. G. (1979) Active site trapping of nucleotides by cross-linking two sulfhydryls in myosin subfragment 1.Proc. natn. Acad. Sci. U.S.A. 76, 4966–70.
Wells, J. A. &Yount, R. G. (1980) Reaction of 5,5′-dithiobis(2-nitrobenzoic acid) with myosin subfragment one: Evidence for formation of a single protein disulfide with trapping of metal nucleotide at the active site.Biochemistry 19, 1711–7.
Wells, J. A. &Yount, R. G. (1982) Chemical modification of myosin by active-site trapping of metal-nucleotides with thiol cross-linking reagents. InMethods in Enzymology (edited byFrederiksen, D. W. andCunningham, L. W.) Vol. 85, pp. 93–116. New York: Academic Press, Inc.
Williams, D. L., Jr. &Greene, L. E. (1983) Comparison of the effects of tropomyosin and troponin-tropomyosin on the binding of myosin subfragment 1 to actin.Biochemistry 22, 2770–4.
Williams, D. L., Jr. &Swenson, C. A. (1982) Disulfide bridges in tropomyosin. Effect on ATPase activity of actomyosin.Eur. J. Biochem. 127, 495–9.
Wray, J. S., Vibert, P. J. &Cohen, C. (1975) Diversity of cross-bridge configurations in invertebrate muscles.Nature, Lond. 257, 561–4.
Yanagida, T. (1981) Angles of nucleotides bound to cross-bridges in glycerinated muscle fiber at various concentrations of ɛ-ATP, ɛ-ADP and ɛ-AMP.PNP detected by polarized fluorescence.J. molec. Biol. 146, 539–60.
Yanagida, T. (1985) Angle of active site of myosin heads in contracting muscle during sudden length changes.J. Musc. Res. Cell Motility 6, 43–52.
Yanagida, T., Kuranaga, I. &Inoué, A. (1982) Interaction of myosin with thin filaments during contraction and relaxation: Effect of ionic strength.J. Biochem., Tokyo 92, 407–12.
Yang, Y.-Z., Korn, E. D. &Eisenberg, E. (1979) Binding of tropomyosin to co-polymers ofAcanthamoeba actin and muscle actin.J. biol. Chem. 254, 2084–8.
Zot, H. G., Güth, K. &Potter, J. D. (1985) Measurement of fluorescence and tension development in skinned skeletal muscle fibers reconstituted with TnCDANZ.Biophys. J. 47, 473a.
Zot, H. G. &Potter, J. D. (1984) The role of calcium in the regulation of the skeletal muscle contraction-relaxation cycle. InMetal Ions in Biological Systems (edited bySiegel, H.), pp. 381–410. New York: Marcel Dekker.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
El-Saleh, S.C., Warber, K.D. & Potter, J.D. The role of tropomyosin-troponin in the regulation of skeletal muscle contraction. J Muscle Res Cell Motil 7, 387–404 (1986). https://doi.org/10.1007/BF01753582
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01753582