Abstract
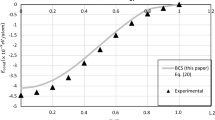
The three-parameter generalized van der Waals equation of state for liquids and gases is analyzed. This equation contains the generalized expressiona/V" for the molecular pressure: here the parametern takes into account the specificity of intermolecular attractive forces for various substances. The equation is presented in the reduced form, from which follows the single-parameter law of corresponding states with the thermodynamic similarity parametern. 11 is established that for alkali metals the value of the parameter it is the same and does not depend on temperature substantially. From the given generalized equation, the expressions for the binodal (equilibrium curve of the liquid and vapor phases) are obtained. For cesium. rubidium, and potassium, the temperature dependence of density is calculated over the temperature range from their melting point to the critical point: the results of the calculations agree with experimental data. It is established that for alkali metals, the law of rectilinear diameter breaks down in the vicinity of the critical point.
Similar content being viewed by others
References
M. M. Martynyuk,Zh. Fiz. Khim. 57:810 (1983).
F. Hensel and H. Uchtmann,Ann. Rev. Phys. Chem. 40:61 (1989).
G. A. Spiridonov and N. V. Kvasov,Obsori Teplofiz. Svoistvam Vesheste 1(57):48 (1986).
M. M. Martynyuk,Zh. Fiz. Khim. 65:1716 (1991).
H. Landolt and R. Bornstein.Zahlenwerte und Funktionen aus Physik-Chemie-Astronomie-Geophysik und Technik. Vol.2. Eigenschaften der materie irr inhren Aggregatzustanden, Part 1.Mechanisch-Thermische Zustandsgrossen (Springer, Berlin, 1971). p. 378.
R. Hultgren, P. D. Desai, and D. T. Hawkins,Selected Values of Thermodynamic Properties of the Elements (American Society for Metals, Metal Park, Ohio, 1973).
K. Hornung, inHandbook o/ Thermodynamic and Transport Properties of Alkali Metals. R. W. Ohse, ed. (IUPAC, Chemical Data #30. Oxford, 1985). pp. 487 524.
P. Browning and P. E. Potter, inHandbook of Thermodynamic and Transport Properties of Alkali Metals, R. W. Ohse, ed. (IUPAC, Chemical Data #30. Oxford, 1985). PP 349 355.
S. Jungst, B. Knuth, and F. Hensel,Phys. Rev. Lett. 55:2160 (1985).
N. B. Vargaltic, E. B. Gelman, V. F. Kozhevnikov, and S. P. Naursakov,Int. J. Thermophys. 11:467 (1990).
M. M. Martynyuk,Parameters of Metal Critical Points (Peoples' Friendship University, Moscow, 1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Martynyuk, M.M., Balasubramanian, R. Equation of state for fluid alkali metals: Binodal. Int J Thermophys 16, 533–543 (1995). https://doi.org/10.1007/BF01441919
Issue Date:
DOI: https://doi.org/10.1007/BF01441919