Summary
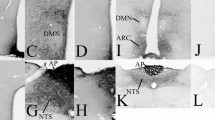
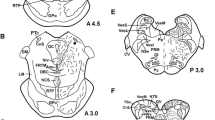
GTP cyclohydrolase I (GCH) is the first and rate-limiting enzyme for the biosynthesis of tetrahydrobiopterin (BH4), the cofactor of phenylalanine, tyrosine, and tryptophan hydroxylases, the enzymes that synthesize tyrosine, catecholamines (dopamine, noradrenaline, and adrenaline), and serotonin, respectively. We produced for the first time polyclonal antibody with highly sensitive immunoreactivity against an oligopeptide of rat enzyme, GFPERELPRPGA, by immunization of rabbits with the peptide conjugated to hemocyanin by glutaraldehyde. The specificity of the antibody was confirmed by Western blot analysis. Using this antibody specific for GCH, we observed strong GCH immunostaining in the liver cells, in the dopamine-, noradrenaline-, adrenaline-, or serotonin-containing cells of the brain, and in the adrenal gland of mice. Immunocytochemical studies revealed GCH to be localized in monoamine-containing perikarya in the periglomerular cells of the olfactory bulb, zona incerta, arcuate nucleus, ventral tegmental area, substantia nigra pars compacta, locus ceruleus, nucleus tractus solitarius, area postrema, and ventrolateral area of the medulla oblongata. GCH immunostaining was particularly strong in serotoninergic nuclei, such as dorsal and median raphe nuclei, nucleus raphe pallidus, and nucleus raphe magnus. By immunoelectron micoscopy, GCH-labeled cytoplasm and microtubules in the processes were observed ultrastructurally, but no staining was found in the mitochondria, and Golgi apparatus. Immunostaining was observed neither in the group D neurons that contain only aromatic amino acid decarboxylase without tyrosine hydroxylase, nor in glial cells and endothelial cells. These results indicate the abundant presence of GCH in catecholaminergic and serotoninergic neurons as well as in the adrenal medulla and liver, where BH4 is synthesized as the cofactor of tyrosine, tryptophan, and phenylalanine hydroxylases.
Similar content being viewed by others
References
Blau N, Thony B, Heizmann CW, Dhondt J-L (1993) Tetrahydrobiopterin efficiency: from phenotype to genotype. Pteridines 4: 1–10
Dinernan JL, Dawson TM, Schell MJ, Snowman A, Snyder SH (1994) Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc Natl Acad Sci USA 91: 4214–4218
Duch DS, Bowers SW, Woolf JH, Nichol CA (1984) Biopterin cofactor biosynthesis: GTP cyclohydrolase, neopterin and biopterin in tissues and body fluids of mammalian species. Life Sci 35: 1895–1901
Hashimoto R, Ozaki N, Ohta T, Kasahara Y, Kaneda N, Nagatsu T (1990) Plasma tetrahydrobiopterin levels in patients with psychiatric disorders. Neuropsychobiology 23: 140–143
Hatakeyama K, Inoue Y, Harada T, Kagamiyama H (1991) Cloning and sequencing of cDNA encoding rat GTP cyclohydrolase I. The first enzyme of the tetrahydrobiopterin biosynthetic pathway. J Biol Chem 266: 765–769
Hirayama K, Lentz SI, Kapatos G (1993) Tetrahydrobiopterin cofactor biosynthesis: GTP cyclohydrolase I mRNA expression in rat brain and superior cervical ganglia. J Neurochem 61: 1006–1014
Hökfelt T, Martensson R, Björklund A, Kleinau S, Goldstein M (1984) Distributional maps of tyrosine-hydroxylase-immunoreactive neurons in the rat brain. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy. Classical transmitters in the CNS, vol 2, part 1. Elsevier, Amsterdam, pp 277–379
Ichinose H, Ohye T, Takahashi E, Seki N, Hori T, Segawa M, Nomura Y, Endo K, Tanaka H, Tsuji S, Fujita K, Nagatsu T (1994) GTP cyclohydrolase I gene as a causative gene for hereditary progressive dystonia with marked diurnal fluctuation. Nature Genet 8: 236–242
Jaeger CB, Ruggier DA, Albert VR, Joh TH, Reis DJ (1984) Immunocytochemical localization of aromatic-L-aminoacid decarboxylase. In: Björklund A, Hökfelt T (eds) Handbook of chemical neuroanatomy. Classical transmitters in the CNS, vol 2, part 1. Elsevier, Amsterdam, pp 387–408
Janssens SP, Shimouchi A, Quertermous T, Bloch DB, Bloch KD (1992) Cloning and expression of a cDNA encoding human endothelium-derived relaxing factor/nitric oxide synthase. J Biol Chem 267: 14519–14522
Kaufman S (1959) Studies on the mechanism of the enzymatic conversion of phenylalanine to tyrosine. J Biol Chem 234: 2677–2682
Kaufman S (1985) Hyperphenylalaninaemia caused by defects in biopterin metabolism. J Inner Metab Dis 8 [Suppl 1]: 20–27
Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu J-Y, Ottersen OP, Storm-Mathisen J, Hama K (1987) Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res 66: 191–210
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685
Lovenberg W, Jequire E, Sjoerdsma A (1967) Tryptophan hydroxylase: measurement in pineal gland, brain stem and carcinoid tumor. Science 155: 217–219
Merrifield WB (1970) Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J Am Chem Soc 85: 2149–2154
Nagatsu I, Kondo Y, Inagaki S, Karasawa N, Kato T, Nagatsu T (1977) Immunofluorescent studies on tyrosine hydroxylase: application for its axoplasmic transport. Acta Histochem Cytochem 10: 494–499
Nagatsu I, Sakai M, Yoshida M, Nagatsu T (1988) Aromatic L-amino acid decarboxylase-immunoreactive neurons in and around the cerebrospinal fluid-contacting neurons of the central canal do not contain dopamine or serotonin in the mouse and rat spinal cord. Brain Res 475: 91–102
Nagatsu I, Kobayashi K, Fujii T, Komori K, Sekiguchi K, Titani K, Fujita K, Nagatsu T (1990a) Antibodies raised against different oligopeptide segments of human dopamine-β-hydroxylase. Neurosci Lett 120: 141–145
Nagatsu I, Komori K, Takeuchi T, Sakai M, Yamada K, Karasawa N (1990b) Transient tyrosine hydroxylase-immunoreactive neurons in the region of the anterior olfactory nucleus of pre- and postnatal mice do not contain dopamine. Brain Res 511: 55–62
Nagatsu I, Yamada K, Karasawa N, Sakai M, Takeuchi T, Kaneda N, Sasaoka T, Kobayashi K, Yokoyama M, Nomura T, Katsuki M, Fujita K, Nagatsu T (1991) Expression in brain sensory neurons of the transgene in transgenic mice carrying human tyrosine hydroxylase gene. Neurosci Lett 127: 91–95
Nagatsu I, Yamada K, Karasawa N, Kaneda N, Sasaoka T, Kobayashi K, Fujita K, Nagatsu T (1993a) Non-catecholaminergic neuronal expression of human tyrosine hydroxylase in the brain of transgenic mice with special reference to aromatic L-amino acid decarboxylase. In: Naoi M, Palvez HS (eds) Tyrosine hydroxylase. VSP Science Press, Zeist The Netherlands, pp 37–57
Nagatsu I, Yamada K, Sakai M, Karasawa N (1993b) Immunocytochemistry and in situ hybridization of catecholamine-synthesizing enzymes and the related neurotransmitters. In: Parvez SH, Naoi M, Nagatsu T, Parvez S (eds) Methods in neurotransmitter and neuropeptide. Elsevier, Amsterdam, pp 151–183
Nagatsu I, Ichinose H, Sakai M, Nagatsu T (1994) Evidence for localization of GTP cyclohydrolase I immunoreactivity in the mouse brain, adrenal gland and liver. Neurosci Res [Suppl 19]: S67 (Abstract)
Nagatsu T, Levitt M, Udenfriend S (1964) Tyrosine hydroxylase: the initial step in norepinephrine biosynthesis. J Biol Chem 239: 2910–2917
Nagatsu T, Yamaguchi T, Kato T, Sugimoto T, Matsuura S, Akino M, Nagatsu I, Iizuka R, Narabayashi H (1981) Biopterin in human brain and urine from controls and parkinsonian patients: application of a new radioimmunoassay. Clin Chim Acta 109: 305–311
Nagatsu T, Sawada M, Yamaguchi T, Sugimoto T, Matsuura S, Akino M, Nakazawa N, Ogawa H (1984) Radioimmunoassay for neopterin in body fluids and tissues. Anal Biochem 141: 472–480
Nagatsu T, Horikoshi T, Sawada M, Nagatsu I, Kondo T, Iizuka R, Narabayashi H (1986) Biosynthesis of tetrahydrobiopterin in Parkinsonian human brain. Adv Neurol 45: 223–226
Nagatsu T, Matsuura S, Sugimoto T (1989) Physiological and clinical chemistry of biopterin. In: deStevens G (ed) Medical research reviews, vol 9, no 1. John Wiley & Sons, New York, pp 25–44
Nathan C, Xie Q (1994) Nitric oxide synthases: roles, tolls, and controls. Cell 78: 915–918
Niederwieser A, Blau N, Wang M, Joller P, Atares M, Cardesa-Garcia J (1984) GTP cyclohydrolase I deficiency, a new enzyme defect causing hyperphenylalaninemia with neopterin, biopterin, dopamine, and serotonin deficiencies and muscular hypotonia. Eur J Pediatr 141: 208–214
Nomura T, Ichinose H, Sumi-Ichinose C, Nomura H, Hagino Y, Fujita K, Nagatsu T (1993) Cloning and sequencing of cDNA encoding mouse GTP cyclohydrolase I. Biochem Biophys Res Commun 191: 523–527
Nygaard TG, Marsden CD, Duvoisin RC (1988) Dopa-responsive dystonia. In: Fahn S, Marsden CD, Calne DB (eds) Advances in neurology, vol 50. Raven Press, New York, pp 377–384
Nygaard TG, Wilhelmsen KC, Risch NJ, Brown DL, Trugman JM, Gilliam TC, Fahn S, Weeks DE (1993) Linkage mapping of dopa-responsive dystonia (DRD) to chromosome 14q. Nature Genet 5: 386–391
Sawada M, Horikoshi T, Masada M, Akino M, Sugimoto T, Matsuura S, Nagatsu T (1986) A sensitive assay of GTP cyclohydrolase I activity in rat and human tissues using radioimmunoassay of neopterin. Anal Biochem 154: 361–366
Sawada M, Hirata Y, Arai H, Iizuka R, Nagatsu T (1987) Tyrosine hydroxylase, tryptophan hydroxylase, biopterin, and neopterin in the brains of normal controls and patients with senile dementia of Alzheimer type. J Neurochem 48: 760–764
Segawa M, Hosaka A, Miyagawa F, Nomura Y, Imai H (1976) Hereditary progressive dystonia with marked diurnal fluctuation. In: Eldridge R, Fahn S (eds) Advances in neurology, vol 14. Raven Press, New York, pp 215–233
Steinbusch HWM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat—cell bodies and terminals. Neuroscience 6: 557–618
Takeuchi Y, Kimura H, Sano Y (1982) Immunohistochemical demonstration of the distribution of serotonin neurons in the brainstem of the rat and cat. Cell Tiss Res 224: 247–267
Togari A, Ichinose H, Matsumoto S, Fujita K, Nagatsu T (1992) Multiple mRNA forms of human GTP cyclohydrolase I. Biochem Biophys Res Commun 187: 359–365
Uchida K, Tsuzaki N, Nagatsu T, Kohsaka S (1992) Tetrahydrobiopterin-dependent functional recovery in 6-hydroxydopamine-treated rats by intracerebral grafting of fibroblasts transfected with tyrosine hydroxylase cDNA. Dev Neurosci 14: 173–180
Werner-Felmayer G, Werner ER, Fuchs D, Hausen A, Reibnegger G, Schmidt K, Weiss G, Wachter H (1993) Pteridine biosynthesis in human endothelial cells. J Biol Chem 268: 1842–1846
Williams AC, Levine RA, Chase TN, Lovenberg W, Calne DB (1980) CFS hydroxylase cofactor levels in some neurological diseases. J Neurol Neurosurg Psychiatry 43: 735–738
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nagatsu, I., Ichinose, H., Sakai, M. et al. Immunocytochemical localization of GTP cyclohydrolase I in the brain, adrenal gland, and liver of mice. J. Neural Transmission 102, 175–188 (1995). https://doi.org/10.1007/BF01281153
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01281153