Abstract
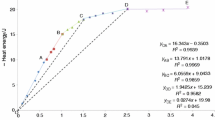
The dissolution rate of multicomponent silicate glasses in a 2.9m aqueous HF solution is investigated as a function of its composition. The glasses studied are composed of SiO2, B2O3, Al2O3, CaO, MgO, ZnO, Na2O and K2O, covering the compositions of most of the technologically important glasses. Unlike many physical properties, no linear relations are observed between the composition of the glass and its dissolution rate. The dissolution rate of a multicomponent silicate glass is found to be largely determined by two factors: The degree of linkage or connectivity of the silicate network and the concentration of SiO2 in the glass. It is proposed that the dissolution of the glasses is preceded by the leaching of alkali and alkaline earth components present in the glass, followed by the subsequent dissolution of the leached layer. Probably fluorine species will diffuse into the leached layer to enhance the dissolution rate. Analysis of the activation energy data indicates that in some corrosive glasses the leaching itself becomes rate determining.
Similar content being viewed by others
References
G. Delapierre,Sensors and Actuators 17 (1989) 123.
J. S. Judge,J. Electrochem. Soc. 118 (1971) 1772.
H. H. Born andM. Prigogine,J. Chem. Phys. 76 (1979) 538.
S. T. Tso andJ. A. Pask,J. Amer. Ceram. Soc. 65 (1982) 360.
A. S. Tenney andM. Ghezzo,J. Electrochem. Soc. 120 (1973) 1091.
Mou-Tion Lee,J. Amer. Ceram. Soc. 67 (1984) C21.
S. Hopland,Mat. Res. Bull. 20 (1985) 1367.
M. Tomozawa andT. Takamori,J. Amer. Ceram. Soc. 62 (1979) 370.
G. A. C. M. Spierings andJ. Van Dijk,J. Mater. Sci. 22 (1987) 1869.
M. Prokopowicz-Prigogine,Glastech. Ber. 62 (1989) 249.
L. Honigmann,ibid. 10 (1932) 154.
R. Brueckner, H.-U. Chun andH. Goretzki,ibid. 51 (1978) 1.
B. M. J. Smets andT. P. A. Lommen,Physics Chem. Glasses 22 (1981) 158.
P. J. Bray, in ‘Borate Glasses’, edited by L. D. Pye, V. D. Frechette and N. J. Kreidl (Plenum Press, New York and London, 1977) pp 321–51.
R. J. Araujo andG. B. Hares,Physics Chem. Glasses 22 (1981) 6.
B. M. J. Smets,Glastechn. Ber. 56K (1983) 1023.
G. A. C. M. Spierings,Physics Chem. Glasses 23 (1982) 101.
H. Scholze, in “Glass: Natur, Structur und Eigenschaften”, (Springer Verlag, Berlin 1977).
D.-T. Liang andD. W. Readey,J. Amer. Ceram. Soc. 70 (1987) 570.
L. K. White,Thin Solid Films 79 (1981) L73.
H. S. Fogler, K. Lund andC. C. McCune,Chem. Eng. Sci. 30 (1975) 1325.
W. E. Kline andH. S. Fogler,Ind. Eng. Chem. Fundam. 20 (1981) 155.
Idem., J. Colloid Inter. Sci. 82 (1981) 93.
Idem., ibid. 82 (1981) 103.
K. Sangwal in “Etching of Crystals; Theory, Experiment, and Application” (North-Holland, The Netherlands, 1987) p. 157.
Z. Boksay, G. Bouquet andS. Dobos,Physics Chem. Glasses 9 (1968) 69.
B. M. J. Smets andT. P. A. Lommen,ibid. 23 (1982) 83.
Idem., J. de Phys. 43 (1982) C9.
L. Chou andR. Wollast,Geochim. Cosmochim. Acta 48 (1984) 2205.
R. W. Douglas andT. M. El-Shamy,J. Amer. Ceram. Soc. 50 (1967) 1.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spierings, G.A.C.M. Compositional effects in the dissolution of multicomponent silicate glasses in aqueous HF solutions. J Mater Sci 26, 3329–3336 (1991). https://doi.org/10.1007/BF01124681
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01124681