Abstract
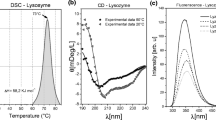
A three-disulfide form of hen egg white lysozyme with Cys6 and Cys127 blocked by carboxymethyl groups was prepared, purified, and characterized for eventual use in protein folding experiments. Trypsin digestion followed by proline-specific endopeptidase digestion facilitated the unambiguous assignment of the disulfide bond pairings and the modified residues in this derivative. 3SS-lysozyme demonstrated nearly full enzymatic activity at itspH optimum,pH 5.5. The 3SS-lysozyme derivative and unmodified lysozyme were shown to be identical by CD spectroscopy atpH 3.6. Immunochemical binding assays demonstrated that the conformation of lysozyme was perturbed predominantly only locally by breaking and blocking the disulfide bond between Cys6 and Cys127. Both 3SS-lysozyme and unmodified lysozyme exhibited reversible thermally induced transitions atpH 2.0 but theT m of 3SS-lysozyme, 18.9°C, was found to be 34° lower than that of native lysozyme under the same conditions. The conformational chemical potential of the denatured form of unmodified lysozyme was determined from the transition curves to be approximately 6.7 kcal/mol higher than that of the denatured form of 3SS-lysozyme, atpH 2.0 and 35°C, if the conformational chemical potential for the folded forms ofboth 3SS-lysozyme and unmodified lysozyme is arbitrarily assumed to be 0.0 kcal/mol. A calculation of the increase in the theoretical loop entropy of denatured 3SS-lysozyme resulting from the cleavage of the Cys6-Cys127 disulfide bond, however, yielded a value of only 5.4 kcal/mol for the difference in conformational chemical potential. This suggests that, in addition to the entropic component, there is also an enthalpic contribution to the difference in the conformational chemical potential corresponding to approximately 1.3 kcal/mol. Thus, it is concluded that the reduction and blocking of the disulfide bond between Cys6 and Cys127 destabilizes 3SS-lysozyme relative to unmodified lysozyme predominantly by stabilizing the denatured conformation by increasing its chain entropy.
Similar content being viewed by others
References
Acharya, A. S., and Taniuchi, H. (1978).Biochemistry 17, 3064–3070.
Acharya, A. S., and Taniuchi, H. (1980).Int. J. Peptide Protein Res. 15, 503–509.
Adler, M., and Scheraga, H. A. (1988).Biochemistry 27, 2471–2480.
Amit, A. G., Mariuzza, R. A., Phillips, S. E. V., and Poljak, R. J. (1986).Science 233, 747–753.
Anfinsen, C. B., and Scheraga, H. A. (1975).Adv. Protein Chem. 29, 205–300.
Atassi, M. Z., Habeeb, A. F. S. A., and Ando, K. (1973).Biochim. Biophys. Acta 303, 203–209.
Blake, C. C. F., Mair, G. A., North, A. C. T., Phillips, D. C., and Sarma, V. R. (1967).Proc. R. Soc. London 167, 365–377.
Canfied, R. E. (1963).J. Biol. Chem. 238, 2691–2697.
Chang, K. Y., and Carr, C. W. (1971).Biochim. Biophys. Acta 229, 496–503.
Chard, T. (1980).Meth. in Enzymol. 70, 280–291.
Chavez, L. G., Jr., and Scheraga, H. A. (1980).Biochemistry 19, 1005–1012.
Colman, P. M., Laver, W. G., Varghese, J. N., Baker, A. T., Tulloch, P. A., Air, G. M., and Webster, R. G. (1987).Nature 326, 358–363.
David, G. S. (1972).Biochem. Biophys. Res. Commun. 48, 464–471.
David, G. S., and Reisfeld, R. A. (1974).Biochemistry 13, 1014–1021.
Davies, D. R., Sheriff, S., Padlan, E. A., Silverton, E. W., Cohen, G. H., and Smith-Gill, S. J. (1989). InThe Immune Response to Structurally Defined Proteins: The Lysozyme Model (Smith-Gill, S. J., and Sercarz, E. E., eds.), Adenine Press, Guilderland, New York, pp. 125–132.
Davies, R. C., Neuberger, A., and Wilson, B. M. (1969).Biochim. Biophys. Acta 178, 294–305.
Denton, M. E., and Scheraga, H. A. (1989). InThe Immune Response to Structurally Defined Proteins: The Lysozyme Model (Smith-Gill, S. J., and Sercarz, E. E., eds.), Adenine Press, Guilderland, New York, pp. 39–40.
Dobson, C. M., and Evans, P. A. (1984).Biochemistry 23, 4267–4270.
Dubois, T., Guillard, R., Prieels, J.-P., and Perraudin, J.-P. (1982).Biochemistry 21, 6516–6523.
Ellman, G. L. (1959).Arch. Biochem. Biophys. 82, 70–77.
Farr, R. S. (1958).J. Infect. Dis. 103, 239–262.
Flory, P. J. (1956).J. Am. Chem. Soc. 78, 5222–5235.
Friguet, B., Chaffotte, A. F., Djavadi-Ohaniance, L., and Goldberg, M. E. (1985).J. Immunol. Methods 77, 305–319.
Furie, B., Schechter, A. N., Sachs, D. H., and Anfinsen, C. B. (1975).J. Mol. Biol. 92, 497–506.
Haeffner-Gormley, L., Parente, L., and Wetlaufer, D. B. (1985).Int. J. Peptide Protein Res. 26, 83–91.
Haeffner-Gormley, L., Poludniak, N. H., and Wetlaufer, D. B. (1981).J. Chromatogr. 214, 185–196.
Hayashi, K., Shimoda, T., Imoto, T., and Funatsu, M. (1968).J. Biochem. 64, 365–370.
Hubbard, A. L., and Cohn, Z. A. (1972).J. Cell Biol. 55, 390–405.
Imoto, T., Johnson, L. N., North, A. C. T., Phillips, D. C., and Rupley, J. A. (1972).The Enzymes, Vol. 7, Academic Press, New York, pp. 665–868.
Johnson, R. E., Adams, P., and Rupley, J. A. (1978).Biochemistry 17, 1479–1485.
Kauzmann, W. (1959). InSulfur in Proteins (Benesch, R., Benesch, R. E., Boyer, P. D., Klotz, I. M., Middlebrook, W. R., Szent-Györgyi, A. G., and Schwarz, D. R., eds.), Academic Press, New York, pp. 93–108.
Konishi, Y., Ooi, T., and Scheraga, H. A. (1982).Biochemistry 21, 4741–4748.
Krohn, K. A., and Welch, M. J. (1974).Int. J. Appl. Radiat. Isot. 25, 315–323.
Lavoie, T. B., Kam-Morgan, L. N. W., Hartman, A. B., Mallett, C. P., Sherriff, S., Saroff, D. A., Mainhart, C. R., Hamel, P. A., Kirsch, J. F., Wilson, A. C., and Smith-Gill, S. J. (1989). InThe Immune Response to Structurally Defined Proteins: The Lysozyme Model (Smith-Gill, S. J., and Sercarz, E. E., eds.), Adenine Press, Guilderland, New York, pp. 151–168.
Lin, S. H., Konishi, Y., Denton, M. E., and Scheraga, H. A. (1984).Biochemistry 23, 5504–5512.
Marchalonis, J. J. (1969).Biochem. J. 113, 299–305.
McWherter, C. A., Thannhauser, T. W., Fredrickson, R. A., Zagotta, M. T., and Scheraga, H. A. (1984).Anal. Biochem. 141, 523–537.
Miller, A., Ch'ng, L.-K., Benjamin, C., Sercarz, E., Brodeur, P., and Riblet, R. (1983).Ann. N.Y. Acad. Sci. 418, 140–150.
Minden, P., and Farr, R. S. (1978). InHandbook of Experimental Immunology, 3rd ed. (Weir, D. M., ed.), Blackwell Scientific Publications, Oxford, England, pp. 13.1–13.22.
O'Reilly, J. M., and Karasz, F. E. (1970).Biopolym. 9, 1429–1435.
Padlan, E. A., Silverton, E. W., Sheriff, S., Cohen, G. H., Smith-Gill, S. J., and Davies, D. R. (1989).Proc. Natl. Acad. Sci. U.S.A. 86, 5938–5942.
Parsons, S. M., and Raftery, M. A. (1972).Biochemistry 11, 1623–1629.
Pfeil, W., and Privalov, P. L. (1976).Biophys. Chem. 4, 33–40.
Phillips, D. C. (1974). InLysozyme (Osserman, E. F., Canfield, R. E., and Beychok, S., eds.), Academic Press, New York, pp. 9–30.
Poland, D. C., and Scheraga, H. A. (1965).Biopolymers 3, 379–399.
Privalov, P. L., and Khechinashvili, N. N. (1974).J. Mol. Biol. 86, 665–684.
Radford, S. E., Woolfson, D. N., Martin, S. R., Lowe, G., and Dobson, C. M. (1991).Biochem. J. 273, 211–218.
Richardson, J. S. (1981).Adv. Protein Chem. 34, 167–339.
Rodbard, D., Bridson, W., and Rayford, P. L. (1969).J. Lab. Clin. Med. 74, 770–781.
Sachs, D. H., Schechter, A. N., Eastlake, A., and Anfinsen, C. B. (1972).Proc. Natl. Acad. Sci. U.S.A. 69, 3790–3794.
Saxena, V. P., and Wetlaufer, D. B. (1970).Biochemistry 9, 5015–5023.
Schellman, J. A. (1955).C.R. Trav. Lab. Carlsberg Ser. Chim. 29, 230–259.
Schwarz, F. P. (1989).Thermochim. Acta 147, 71–91.
Sheriff, S., Silverton, E. W., Padlan, E. A., Cohen, G. H., Smith-Gill, S. J., Finzel, B. C., and Davies, D. R. (1987).Proc. Natl. Acad. Sci. U.S.A. 84, 8075–8079.
Shugar, D. (1952).Biochim. Biophys. Acta 8, 302–309.
Smith-Gill, S. J., Laboie, T. B., and Mainhart, C. R. (1984).J. Immunol. 133, 384–393.
Smith-Gill, S. J., Wilson, A. C., Potter, M., Prager, E. M., Feldmann, R. J., and Mainhart, C. R. (1982).J. Immunol. 128, 314–322.
Sophianopoulos, A. J., and Weiss, B. J. (1964).Biochemistry 3, 1920–1928.
Thannhauser, T. W., Konishi, Y., and Scheraga, H. A. (1984).Anal. Biochem. 138, 181–188.
Thannhauser, T. W., McWherter, C. A., and Scheraga, H. A. (1985).Anal. Biochem. 149, 322–330.
Thorell, J. I., and Johansson, B. G. (1971).Biochim. Biophys. Acta 251, 363–369.
Thornton, J. M. (1981).J. Mol. Biol. 151, 261–287.
Ueda, T., Yamada, H., Hirata, M., and Imoto, T. (1985).Biochemistry 24, 6316–6322.
Walsh, K. A. (1970).Methods Enzymol. 19, 41–63.
Wang, M. C., and Uhlenbeck, G. E. (1945).Rev. Mod. Phys. 17, 323–342.
Yalow, R. S., and Berson, S. A. (1970). InStatistics in Endocrinology (McArthur, J. W., and Colton, T., eds.), MIT Press, Cambridge, Massuchasetts, pp. 327–344.
Yamada, H., Imoto, T., Fujita, K., Okazaki, K., and Motomura, M. (1981).Biochemistry 20, 4836–4842.
Yamada, H., Kuroki, R., Hirata, M., and Imoto, T. (1983).Biochemistry 22, 4551–4556.
Author information
Authors and Affiliations
Additional information
Cornell Biotechnology Army Research Office Predoctoral Fellow, 1986–1989.
Rights and permissions
About this article
Cite this article
Denton, M.E., Scheraga, H.A. Spectroscopic, immunochemical, and thermodynamic properties of carboxymethyl(Cys6, Cys127)-hen egg white lysozyme. J Protein Chem 10, 213–232 (1991). https://doi.org/10.1007/BF01024786
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01024786