Abstract
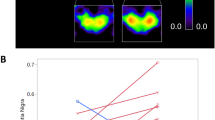
A woman affected by chronic progressive external ophthalmoplegia and muscle mitochondrial DNA deletion was studied by phosphorus magnetic resonance spectroscopy (31P-MRS) prior to and after 1 and 7 months of treatment with oral lipoic acid. Before treatment a decreased phosphocreatine (PCr) content was found in the occipital lobes, accompanied by normal inorganic phosphate (Pi) level and cytosolic pH. Based on these findings, we found a high cytosolic adenosine diphosphate concentration [ADP] and high relative rate of energy metabolism together with a low phosphorylation potential. Muscle MRS showed an abnormal work-energy cost transfer function and a low rate of PCr recovery during the post-exercise period. All of these findings indicated a deficit of mitochondrial function in both brain and muscle. Treatment with 600 mg lipoic acid daily for 1 month resulted in a 55% increase of brain [PCr], 72% increase of phosphorylation potential, and a decrease of calculated [ADP] and rate of energy metabolism. After 7 months of treatment MRS data and mitochondrial function had improved further. Treatment with lipoate also led to a 64% increase in the initial slope of the work-energy cost transfer function in the working calf muscle and worsened the rate of PCr resynthesis during recovery. The patient reported subjective improvement of general conditions and muscle performance after therapy. Our results indicate that treatment with lipoate caused a relevant increase in levels of energy available in brain and skeletal muscle during exercise.
Similar content being viewed by others
References
Argov Z, Bank WJ, Maris J, Chance B (1987) Muscle energy metabolism in McArdle's syndrome by in vivo phosphorus magnetic resonance spectroscopy. Neurology 37:1720–1724
Argov Z, Bank WJ, Maris J, Peterson P, Chance B (1987) Bioenergetic heterogeneity of human mitochondrial myopathies: phosphorus magnetic resonance spectroscopy study. Neurology 37:257–262
Arnold DL, Matthews PM, Radda GK (1984) Metabolic recovery after exercise and the assesment of mitochondrial function in human skeletal muscle in vivo by means of 31P-NMR. Mag Res Med 1:307–315
Arnold DL, Taylor DJ, Radda GK (1985) Investigation of human mitochondrial myopathies by phosphorus magnetic resonance spectroscopy. Ann Neurol 18:189–196
Barbiroli B, Montagna P, Cortelli P, Martinelli P, Sacquegna T, Zaniol P, Lugaresi E (1990) Complicated migraine studied by phosphorus magnetic resonance spectroscopy. Cephalalgia 10:263–272
Barbiroli B, Funicello R, Ferlini A, Montagna P, Zaniol P (1992) Muscle energy metabolism in female DMD/BMD carriers: a 31P-MR study. Muscle Nerve 15:344–348
Barbiroli B, Montagna P, Cortelli P, Funicello R, Iotti S, Monari L, Pierangeli G, Zaniol P, Lugaresi E (1992) Abnormal brain and muscle eneigy metabolism shown by31P magnetic resonance spectroscopy in patients affected by migraine with aura. Neurology 42:1209–1214
Barbiroli B, Montagna P, Martinelli P, Lodi R, Iotti S, Cortelli P, Funicello R, Zaniol P (1993) Defective brain energy metabolism shown by in vivo 31P MR spectroscopy in 28 patients with mitochondrial cytopathies. J Cereb Blood Flow Metab 13:469–474
Bendaham D, Confort-Gouny S, Kozak-Reiss G, Cozzone P (1992) 31P NMR characterization of the metabolic anomalies associated with the lack of glycogen phosphorylase activity in human forearm muscle. Biochem Biophys Res Commun 185:16–21
Bendahan D, Desnuelle C, Vanuxem D, Confort-Gouny S, Figarella-Branger D, Pellissier JF, Kozak-Ribbens G, Pouget J, Serratrice G, Cozzone PJ (1992)31P NMR spectroscopy and ergometer test as evidence for muscle oxidative performance improvement with coenzyme Q in mitochondrial myopathies. Neurology 42:1203–1208
Bottomly PA, Hardy CJ (1989) Rapid, reliable in vivo assay of human phosphate metabolites by nuclear magnetic resonance. Clin Chem 59:392–395
Bottomly PA, Foster TH, Darrow RD (1984) Depth-resolved surface-coil spectroscopy (DRESS) for in vivo1H,31P, and13C NMR. J Magn Reson 59:338–342
Chance B, Leigh JS Jr, Clark BJ, Maris J, Kent J, Nioka S, Smith D (1985) Control of oxidative phosphorylation and oxygen delivery in human skeletal muscle: a steady-state analysis of the work-energy cost transfer funtion. Proc Natl Acad Sci USA 83:8384–8388
Chance B, Leigh JS, Kent J, McCully KK, Nioka S, Clark BJ, Maris JM, Graham T (1986) Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci USA 83:9458–9462
Clark BJ, Smith B, Chance B (1987) Metabolic consequences of oxygen transport studied with phosphorus nuclear magnetic resonance spectroscopy. In: Bryan-Vrown CW, Ayres SM (eds) Oxygen transport and utilization. Society Critical Care Medicine, Fullerton, Calif, pp 144–170
Clayton BE, Dobbs RH, Patrick AD (1967) Leigh's subacute necrotizing encephalopathy: clinical and biochemical study with special reference to therapy with lipoate. Arch Dis Childr 42:467–472
Cortelli P, Montagna P, Avoni P, Sangiorgi S, Bresolin N, Moggio M, Zaniol P, Mantovani V, Barboni P, Barbiroli B, Lugaresi E (1991) Leber's hereditary optic neuropathy: genetic, biochemical and phosphorus magnetic resonance spectroscopy study in an Italian family. Neurology 41:1211–1215
Crome L, Stern J (1967) The pathology of mental retardation. Churchill, London, p 314
De Certains JD, Bovee WMMJ, Podo F (eds) (1992) Magnetic resonance spectroscopy in biology and medicine. Pergamon Press, Oxford
Delivoria-Papadopulos M, Chamce B (1988) In: Guthrie RD (ed) Neonatal Intensive care. Churchill Livingstone, New York, pp 153–179
Di Mauro S, Bonilla E, Zeviani M, Nakagawa M, De Vivo DC (1985) Mitochondrial myopathics. Ann Neurol 17:521–538
Duboc D, Jehenson P, Tran Din S, Marsac C, Syrota A, Fardeau M (1987) Phosphorus NMR spectroscopy study of muscular enzyme deficiencies involving glycogenolysis and glycolysis. Neurology 37:663–671
Dubowitz V (1985) Muscle Biopsy: a practical approach. Bailliere Tindall, Eastbourne
EEC Concerted Research Project (1995) Quality assessment in in vivo NMR spectroscopy. II. A protocol for quality assessment. Magn Reson Imaging 13 (in press)
Eleff S, Kennaway NG, Buist NG, Darley-Usmar VM, Capaldi RA, Bank WJ, Chance B (1984)31P NMR study of improvement in oxidative phosphorylation by vitamin K3 and C in a patient with a defect in electron transport at complex III in skeletal muscle. Proc Natl Acad Sci USA 81:3529–3533
Eleff SM, Barker PB, Blackband SJ, Chathman JC, Lutz NW, Johns DR, Bryan RN, Hurko O (1990) Phosphorus magnetic resonance spectroscopy of patients with mitochondrial cytopathies demonstrates decreased levels of brain phosphocreatine. Ann Neurol 27:626–630
Haugaard N, Haugaard ES (1970) Stimulation of glucose utilization by thioctic acid in rat diphragm in vitro. Biochim Biophys Acta 222:583–586
Hommes FA, Polman HA, Reevink JD (1968) Leigh's encephalomyopathy: an inborn error of gluconeogenesis. Arch Dis Child 43:423–426
Iotti S, Lodi R, Frassineti C, Zaniol P, Barbiroli B (1993) In vivo assessment of mitochondrial functionality in human gastrocnemius muscle by 31P MRS. The role of pH in the evaluation of phosphocreatine and inorganic phosphate recoveries from exercise. NMR Biomed 6:248–243
Maesaka H, Komiya K, Misugi K, Tada K (1976) Hyperalaninemia, hyperpyruvicemia and lactic acidosis due to pyruvate carboxylase deficiency of the liver; treatment with thiamine and lipoic acid. Eur J Pediatr 122:159–168
Matalon R, Stumpf DA, Kimberlee M, Hart RD, Parks JK Goodman SJ (1984) Lipoamide dehydrogenase deficiency with primary lactic acidosis: favorable response to treatment with oral lipoic acid. J Pediatr 104:65–69
Matthews PM, Allaire C, Shoubridge EA, Karpati G, Carpenter S, Arnold DL (1991) In vivo muscle magnetic resonance spectroscopy in the clinical investigation of mitochondrial disease. Neurology 41:114–120
Matthews PM, Ford B, Dandurand RJ, Eidelman DH, O'Connor D, Sherwin A, Karpati G, Andermann F, Arnold DL (1993) Coenzyme Q10 with multiple vitamins is generally ineffective in treatment of mitochondrial diseases. Neurology 43:884–890
Montagna P, Gallassi R, Medori R, Govoni E, Zevianoi S, DiMauro S, Lugaresi E, Anderman F (1988) MELAS syndrome: characteristic migrainous epileptic features and maternal transmission. Neurology 38:751–754
Montagna P, Martinelli P, Cortelli P, Zaniol P, Lugaresi E, Barbiroli B (1992) Brain31P-magnetic resonance spectroscopy in mitochondrial cytopathies. Ann Neurol 31:451–452
Natraj CV, Gandhi VM, Menon KKG (1984) Lipoic acid and diabetes: effect of dihydrolipoic acid administration in diabetic rats and rabbits. J Biosci 6:37–46
Nishikawa Y, Takahashi M, Yorifuji S, Nakamura Y, Ueno S, Tarui S, Kozuka T, Nishimura T (1989) Long-term coenzyme Q10 therapy for a mitochondrial encephalopathy with cytochrome C oxidase deficiency: a31P NMR study. Neurology 39:399–403
Pernow B, Saltin B (1971) Availability of substrates and capacity for prolonged heavy exercise in man. J Appl Physiol 31:416–422
Petroff OAC, Prichard JW, Behar KL, Alger JR, Shulman RG (1985) Cerebral metabolism in hyper- and hypocarbia: 31P and 1H nuclear magnetic resonance studies. Neurology 35:1681–1688
Przyrembel H (1987) Therapy of mitochondrial disorders. J Inherit Metab Dis 10:129–146
Rice JE, Dunhar B, Lindsay JG (1992) Sequences directing dihydrolipoamide dehydrogenase (E3) binding are located on the 2-oxoglutarate dehydrogenase (E1) component of the mammalian 2-oxoglutarate dehydrogenase multienzyme complex. EMBO J 11:3229–3235
Tritschler HJ, Andreetta F, Moraes C, Bonilla E, Amaudo E, Danon MJ, Glass S, Zeyala BM, Vamos E, Teleman-Toppet N, DiMauro S, Schon EA (1992) Mitochondrial myopathy of childhood associated with depletion of mitochondrial DNA. Neurology 42:209–217
Veech RL, Lawson JWR, Cornell NW, Krebs HA (1979) Cytosolic phosphorylation potential. J Biol Chem 354:6538–6547
Wagh SS, Natraj CV, Menon KKG (1987) Mode of action of lipoic acid in diabetes. J Biosci 11:59–74
Wallace DC, Zheng X, Lott MT, Shoffner JM, Hodge JA, Kelly RI, Epstein CM, Hopkins LC (1988) Familial mitochondrial encephalomyopathy (MERRF): genetic, pathophysiological, and biochemical characterization of mitochondrial DNA disease. Cell 55:601–610
Zaniol P, Serafini M, Ferraresi M, Golinelli R, Bassoli P, Canossi I, Aprilesi GC, Barbiroli B (1992) Muscle31P-MR spectroscopy performed routinely in a clinical environment by a wholebody imager/spectrometer. Phys Med 8:87–91
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barbiroli, B., Medori, R., Tritschler, H.J. et al. Lipoic (thioctic) acid increases brain energy availability and skeletal muscle performance as shown by in vivo31P-MRS in a patient with mitochondrial cytopathy. J Neurol 242, 472–477 (1995). https://doi.org/10.1007/BF00873552
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00873552