Summary
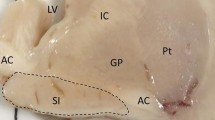
The deficiency of the cholinergic cortical projection system arising in the different basal forebrain structures collectively referred to as nucleus basalis of Meynert complex is a constant finding in Alzheimer's disease, a disorder which is neuropathologically characterised by the appearance of three intracerebral formes of twisted β-pleated sheet (amyloid) fibrils, neurofibrillary tangles, amyloid-containing neuritic plaques and congophilic amyloid angiopathy. In the present study the quantitative relationship between these hallmarks of the disease, amyloid deposition and neuronal loss in the cholinergic basal forebrain system, was investigated in ten cases of Alzheimer's disease. Besides a constant involvement of the cerebral cortex and hippocampus, all cases of Alzheimer's disease show a large amount of amyloid in the medial septal nucleus, in the diagonal band nucleus and in the substantia innominata which is correlated with neuronal loss in these areas. These amyloid deposits in the basal forebrain are due to congophilic angiopathy associated with plaques and neurofibrillary tangles. The distribution of amyloid deposition in the basal forebrain is restricted entirely to those neuronal clusters which represent the origin of cholinergic innervation of the cerebral cortex and hippocampus. Immediately adjacent structures are not affected. These findings suggest a pathogenetic role of amyloid deposition in the mechanism of degeneration of the cholingeric basal forebrain system.
Similar content being viewed by others
References
Alzheimer A (1904) Histologische Studien zur Differentialdiagnose der progressiven Paralyse. Nissls Arb 1:18
Alzheimer A (1907) Über eine eigenartige Erkrankung der Hirnrinde. Allg Z Psychiatr 64:146–148
Arendt T, Bigl V (1986) Alzheimer plaques and cortical cholinergic innervation. Neuroscience 17:277–279
Arendt T, Bigl V, Arendt A, Tennstedt A (1983) Loss of neurons in the nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans and Korsakoff's disease. Acta Neuropathol (Berl) 61:101–108
Arendt T, Bigl V, Tennstedt A, Arendt A (1985) Neuronal loss in different parts of the nucleus basalis is related to neuritic plaque formation in cortical areas in Alzheimer's disease. Neuroscience 14:1–14
Bielschowsky M (1911) Zur Kenntnis der Alzheimerschen Krankheit (präsenilen Demenz mit Herdsymptomen). J Psychol Neurol 18:273–292
Blessed G, Tomlinson BE, Roth (1968) The association between quantitative measures of dementia and of senile change in the gray matter of elderly subjects. Br J Psychiatry 114:797–811
Bondareff W, Mountjoy CQ, Roth M (1981) Selective loss of neurones of origin of adrenergic projection to cerebral cortex (nucleus locus coeruleus) in senile dementia. Lancet I:783–784
Burger PC, Vogel FS (1973) The development of the pathologic changes of Alzheimer's disease and senile dementia in patients with Down's syndrome. Am J Pathol 73:457–476
Corsellis JAN, Bruton CJ, Freeman-Browne D (1973) The aftermath of boxing. Psychol Med 3:270–303
Divac J (1975) Magnocellular nuclei of the basal forebrain project to neocortex, brain stem and olfactory bulb. Review of some functional correlates. Brain Res 93:385–398
Divry P (1927) Etude histochimique des plaques seniles. J Belge Neurol Psychiatr 27:643–657
Divry P (1934) De la nature de l'alteration fibrillaire d'Alzheimer. J Belge Neurol Psychiatr 34:197–201
Eanes ED, Glenner GG (1968) X-ray diffraction studies of amyloid filaments. J Histochem Cytochem 16:673–805
Fischer O (1907) Miliare Nekrosen mit drusiger Wucherung. Monatsschr Psychiatr Neurol 22:361
Glenner GG, Wong CW (1984) Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun 122:1131–1135
Hirano A, Zimmerman HM (1962) Alzheimer's neurofibrillary changes: a topographic study. Arch Neurol 7:227–242
Hirano A, Malamud N, Kurland LT (1961) Parkinsonism-dementia complex, endemic disease on the island of Guam. 2. Pathological features. Brain 84:662–679
Ishii T (1966) Distribution of Alzheimer's neurofibrillary changes in the brain stem and hypothalamus of senile dementia. Acta Neuropathol (Berl) 6:181–187
Kidd M, Allsop D, Landon M (1985) Senile plaque amyloid paired helical filaments and cerebrovascular amyloid in Alzheimer's disease are all deposits of the same protein. Lancet I:278
MacDonald SM, Esiri MM (1986) Monoclonal antibody binding to congophilic elements in human Alzheimer brain. J Clin Pathol 39:1199–1203
Mandybur TI (1967) The incidence of cerebral amyloid angiopathy in Alzheimer's disease. Neurology 25:120–125
Mann DMA, Yates PO, Hawkes J (1982) The noradrenergic system in Alzheimer and multiinfarct dementias. J Neurol Neurosurg Psychiatry 45:113–119
Mann DMA, Yates PO, Marcyniuk B (1984) Alzheimer's presenile dementia, senile dementia of Alzheimer's type and Down's syndrome in middle age form an age-related continuum of pathological changes. Neuropathol Appl Neurobiol 10:185–207
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer's disease and Down syndrome. Proc Natl Acad Sci USA 82:4245–4249
Masters CL, Multhaup G, Simms G, Pottgiesser J, Martins RN, Beyreuther K (1985) Neuronal origin of a cerebral amyloid: neurofibrillary tangles of Alzheimer's disease contain the same protein as the amyloid of plaque cores and blood vessels. EMBO J 4:2757–2763
Mesulam MM, van Hoesen GW (1976) Acetylcholinesterase-rich projections from the basal forebrain of the rhesus monkey to neocortex. Brain Res 109:152–157
Miyakawa T, Shimoji A, Kuramato R, Higuchi Y (1982) The relationship between senile plaques and cerebral blood vessels in Alzheimer's disease and senile dementia. Virchows Arch [B] 40:121–129
Nakano I, Hirano A (1983) Neuron loss in the nucleus basalis of Meynert in Parkinsonism-dementia complex of Guam. Ann Neurol 13:87–91
Nakashima S, Ikuta F (1985) Catecholamine neurons with Alzheimer's neurofibrillary changes and alteration of tyrosine hydroxylase. Acta Neuropathol (Berl) 66:37–41
Romhanyi G (1971) Selective differentiation between amyloid and connective tissue structures based on the collagen specific topo-optical staining reaction with congo red. Virchows Arch [A] 354:209–222
Rudelli RD, Ambler MW, Wisniewski HM (1984) Morphology and distribution of Alzheimer neuritic (senile) and amyloid plaques in striatum and diencephalon. Acta Neuropathol (Berl) 64:273–281
Saper CB, German DC, White CL III (1985) Neuronal pathology in the nucleus basalis and associated cell groups in senile dementia of the Alzheimer's type: possible role in cell loss. Neurology 35:1089–1095
Schwartz P (1965) New aspects of presenile and senile deterioration. A new triad: cerebral, cardiovascular and pancreatic insular amyloidosis of the aged. Exhib 19th Clinical Convention Amercian Medical Association, Philadelphia, Pennsylvania
Schwartz P (1967) Neue Beiträge zur Pathologie des Alterns. Psychiat Neurol 154:337–365
Schwartz P (1967) Systemic amyloid degeneration in mongoloid idiocy. J Neuropathol Exp Neurol 26:149–150
Schwartz P (1970) Amyloidosis: cause and manifestations of senile deterioration. Thomas, Springfield
Shortridge BA, Vogel FS, Burger PC (1985) Topographic relationship between neurofibrillary change and acetylcholinesterase rich neurons in the upper brain stem of patients with senile dementia of the Alzheimer's type and Down's syndrome. Clin Neuropathol 4:227–237
Smith JS (1981) The investigation of dementia: results in 200 consecutive admissions. Lancet I:824–827
Surbeck KB (1961) L'angiopathie dyshorique (Morel) de l'ecorce cerebrale. Etude anatomoclinique et statistique: aspect genetique. Thesis, Geneve
Suzuki K, Terry RD (1967) Fine structural localization of acid phosphatase in senile plaques in Alzheimer's presenile dementia. Acta Neuropathol (Berl) 8:276–284
Uhl GR, McKinney M, Hedreen JC, White III CL, Coyle JT, Whitehouse PJ, Price DL (1982) Dementia pugilistica: loss of basal forebrain cholinergic neurons and cortical cholinergic markers. Ann Neurol 12:99
Wells CE (1978) Chronic brain disease: an overview. Am J Psychiatry 135:1–12
Wenk H, Bigl V, Meyer U (1980) Cholinergic projections from magnocellular nuclei of the basal forebrain to cortical areas in rats. Brain Res Rev 2:295–316
Whitehouse PJ, Price DL, Stuble RG, Clark AW, Coyle JT, DeLong MR (1982) Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science 215:1237–1239
Wilcock GK, Esiri MM (1982) Plaques, tangles and dementia. J Neurol Sci 56:343–356
Yamamoto T, Hirano A (1985) Nucleus raphe dorsalis in Alzheimer's disease: neurofibrillary tangles and loss of large neurons. Ann Neurol 17:573–577
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arendt, T., Taubert, G., Bigl, V. et al. Amyloid deposition in the nucleus basalis of Meynert complex: a topographic marker for degenerating cell clusters in Alzheimer's disease. Acta Neuropathol 75, 226–232 (1988). https://doi.org/10.1007/BF00690530
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00690530