Abstract
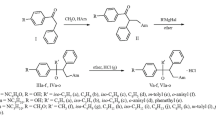
Analogues of 3-aminooxy-1-propanamine proved to be highly potent and selective inhibitors of ornithine decarboxylase (ODC). The compounds competed with ornithine for the substrate binding site of ODC, but resulted in progressive and apparently irreversible inactivation of the enzyme. Diamine oxidase was inhibited by these compounds to a lesser extent than ODC; the compounds were not metabolized by this enzyme. Several derivatives were growth-inhibitory for human T24 cells and for other mammalian cells, the most active compound being 3-aminooxy-2-fluoro-1-propanamine (AFPA). Growth-arrested cells were largely depleted of putrescine and spermidine. Cellular growth arrest could be antagonized by supplementation with spermidine. Selection for resistance against AFPA led to cells with amplified ODC genes and overexpression of the message. Some of the derivatives were tumoristatic at well-tolerated doses in mice bearing solid T24 tumours. The antiproliferative activity of these compounds appears to be mediated by polyamine depletion.
Similar content being viewed by others
References
Abdel-Monem MM, Newton NE, Weeks C (1974) Inhibitors of polyamine biosynthesis. 1. Alpha-methyl-(+)-ornithine, an inhibitor of ornithine decarboxylase. J Med Chem 17: 447
Bowlin TL, Hoeper BJ, Rosenberger AL, Davis GF, Sunkara PS (1990) Effects of three irreversible inhibitors of ornithine decarboxylase on macrophage-mediated tumoricidal activity and antitumor activity in B16F1 tumor-bearing mice. Cancer Res 50: 4510
Bradford MM (1976) A rapid sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248
Forastiere AA, Natale RB, Wheeler RR (1986) Phase II trial of methylglyoxal bis(guanylhydrazone) (MGBG) in advanced head and neck cancer. Cancer 58: 2585
Hayashi S, Kameji T (1983) Ornithine decarboxylase (rat liver), In. Tabor H, White-Tabor C (eds) Methods in enzymology, vol 94: Polyamines. Academic Press, New York, p 154
Horn Y, Schechter PJ, Marton LJ (1987) Phase I–II clinical trial with alpha-difluoromethylornithine, an inhibitor of polyamine biosynthesis. Eur J Cancer Clin Oncol 23: 1103
Hyvönen T, Eloranta TO (1990) Regulation ofS-adenosyl-l-methionine decarboxylase by 1-aminooxy-3-aminopropane: enzyme kinetics and effects on the enzyme activity in cultured cells. J Biochem 107: 339
Hyvönen T, Alakuijala L, Andersson L, Khomutov AR, Khomutov RM, Eloranta TO (1988) I-Aminooxy-3-aminopropane reversibly prevents the proliferation of cultured baby hamster kidney cells by interfering with polyamine synthesis. J Biol Chem 263: 11138
Hyvönen T, Khomutov AR, Khomutov RM, Lapinjoki S, Eloranta TO (1990) Uptake of3H-labeled 1-aminooxy-3-aminopropane by baby hamster kidney cells. J Biochem 107: 817
Kabra PM, Lee HK, Lubich WP, Marton LJ (1986) Solid-phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr 380: 19
Kendrick DA, Danzin C, Kolb M (1989) 2,2-Difluoro-5-hexyne-1,4-diamine: a potent enzyme-activated inhibitor of ornithine decarboxylase. J Med Chem 32: 170
Khomutov RM, Hyvönen T, Karvonen E, Kauppinen L, Paalanen T, Paulin L, Eloranta TO, Pajula R-L, Andersson LC, Pösö H (1985) I-Aminooxy-3-aminopropane, a new and potent inhibitor of polyamine biosynthesis that inhibits ornithine decarboxylase, adenosylmethionine decarboxylase and spermidine synthase. Biochem Biophys Res Commun 130: 596
Kopitz J, Adam G, Bohley P (1990) Very fast purification of ornithine decarboxylase with high yield from mouse kidney and generation of a monoclonal antibody. Biol Chem Hoppe Seyler 371: 363
Lapinjoki S, Eloranta TO, Pösö H (1987) On the mechanism of ornithine decarboxylase inhibition by 3-aminooxypropylamine. In: Korpela T, Christen P (eds) Biochemistry of vitamin B6. Birkhäuser, Basel, p 321
Leinonen P, Alhonen-Hongisto L, Laine R, Jänne OA, Jänne J (1987) Human myeloma cells acquire resistance to difluoromethylornithine by amplification of ornithine decarboxylase gene. Biochem J 242: 199
Lipton A, Harvey HA, Glenn J, Weidner WA, Strauss M, Miller SE, Taylor JB, White-Hershey D, Barlow JLR (1989) A phase I study of hepatic arterial infusion using difluoromethylornithine. Cancer 63: 433
Maddox AM, Freireich EJ, Keating MJ, Haddox MK (1988) Alterations in bone marrow and blood mononuclear cell polyamine and methylglyoxal bis(guanylhydrazone) levels: phase I evaluation of alpha-difluoromethylornithine and methylglyoxal bis(guanylhydrazone) treatment of human hematological malignancies. Cancer Res 48: 1367
Mamont PS, Duchesne M-C, Grove J, Bey P (1978) Antiproliferative properties ofDL-alpha-difluoromethylornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun 81: 58
Meyer T, Regenass U, Fabbro D, Alteri E, Rösel J, Müller M, Caravatti G, Matter A (1989) A derivative of staurosporine (CGP 41251) shows selectivity for protein kinase C inhibition and in vitro antiproliferative as well as in vivo anti-tumor activity. Int J Cancer 43: 851
Nagarajan S, Ganema B, Pegg AE (1988) Studies of non-metabolizable polyamines that support growth of SV-3T3 cells depleted of natural polyamines by exposure to alpha-difluoromethylornithine. Biochem J 254: 373
Nicolet TG, Scemama J-L, Pradayrol L, Seva C, Vaysse N (1990) Characterization of putrescine and spermidine transport systems of a rat pancreatic acinar tumor cell line (AR4-2J). Biochem J 269: 629
Pegg AE (1986) Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J 234: 249
Pegg AE (1988) Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy. Cancer Res 48: 759
Pegg AE, Jones DB (1988) Effect of inhibitors ofS-adenosylmethionine decarboxylase on polyamine content and growth of L1210 cells. Biochemistry 27: 1408
Pegg AE, Pösö H (1983)S-Adenosylmethionine decarboxylase (rat liver). In: Tabor H, White-Tabor C (eds) Methods in enzymology, vol 94: Polyamines. Academic Press, New York, p 234
Pegg AE, Madhubala R, Kameji T, Bergeron RJ (1988) Control of ornithine decarboxylase activity in alpha-difluoromethylornithine-resistant L1210 cells by polyamines and synthetic analogs. J Biol Chem 263: 11008
Pleshkewych A, Kramer DL, Kelly E, Porter CW (1980) Independence of drug action on mitochondria and polyamines in L1210 leukemia cells treated with methylglyoxal bis(guanylhydrazone). Cancer Res 40: 4533
Porter CW, McManis J, Casero RA, Bergeron RJ (1987) Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res 47: 2821
Poulin L, Secrist JA III, Pegg AE (1989) Effect of 1-amino-oxy-3-aminopropane on polyamine metabolism and growth of L1210 cells. Biochem J 263: 215
Poulin R, Lu L, Ackermann B, Bey P, Pegg AE (1992) Mechanism of irreversible inactivation of mouse ornithine decarboxylase by alpha-difluoromethylornithine. Characterization of sequences at the inhibitor and coenzyme binding sites. J Biol Chem 267: 150
Seely JE, Pegg AE (1983) Ornithine decarboxylase (mouse kidney). In: Tabor H, White-Tabor C (eds) Methods in enzymology, vol 94: Polyamines. Academic Press, New York, p 158
Seiler N (1990) Polyamine metabolism. Digestion 46 [Suppl 2]: 319
Seppänen P, Alhonen-Hongisto L, Jänne J (1980) Relation of the antiproliferative action of methylglyoxal bis(guanylhydrazone) to the natural polyamines. Eur J Biochem 110: 7
Seppänen P, Alhonen-Hongisto L, Käpyaho K, Jänne J (1983) Two enzyme inhibition assays for methylglyoxal bis(guanylhydrazone). In: Tabor H, White-Tabor C (eds) Methods in enzymology, vol 94: Polyamines. Academic Press, New York, p 247
Stanek J, Frei J, Mett H, Schneider P, Regenass U (1992) 2-Substituted 3-(aminooxy)propanamines as inhibitors of ornithine decarboxylase: synthesis and biological activity. J Med Chem 35: 1339
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mett, H., Stanek, J., Lopez-Ballester, J.A. et al. Pharmacological properties of the ornithine decarboxylase inhibitor 3-aminooxy-1-propanamine and several structural analogues. Cancer Chemother. Pharmacol. 32, 39–45 (1993). https://doi.org/10.1007/BF00685874
Issue Date:
DOI: https://doi.org/10.1007/BF00685874