Abstract
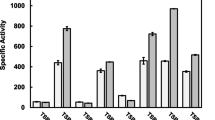
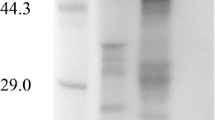
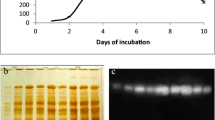
β-Glucosidase released by the phytoflagellate Ochromonas danica was the result of secretion; this was adduced from the following: (1) The enzyme was released during growth, including early log phase. (2) The amount released was calculated to be much more than could be attributed to cell lysis. (3) β-Glucosidase was released by cells during short term incubation in a dilute salt solution; this release was nearly linear for at least 24 h. (4) Release occurred while cell counts remained nearly constant and cells remained viable. (5) Control experiments excluded cell damage resulting from incubation and cell manipulation as a source of the exoenzyme. (6) No alkaline phosphatase was released and 5 times less phosphoglucose isomerase than glucosidase was released while the cells contained 7 times more phosphoglucose isomerase. (7) The kinetics of release of nonspecific protein and UV absorbing material was markedly different from glucosidase release. (8) Glucosidase release was temperature and energy dependent; anaerobiosis decreased enzyme release. (9) Release was inhibited by cycloheximide. (10) Cells incubated with 3H-leucine synthesized labeled protein which was secreted linearly for at least 24h. Cycloheximide inhibited incorporation of 3H-leucine into protein and the secretion of the labeled protein.
Similar content being viewed by others
Abbreviations
- CHI:
-
cycloheximide
- DNP:
-
2,4-dinitrophenol
- IAA:
-
iodoacetic acid
- PGI:
-
phosphoglucose isomerase
- SIS:
-
salt incubation solution
References
Aaronson, S.: Digestion in phytoflagellates. In: Lysosomes in biology and pathology, Vol. 3 (J. T. Dingle, H. B. Fell, eds.), pp. 18–37. London: North-Holland 1973
Aaronson, S., Baker, H.: A comparative biochemical study of two species of Ochromonas. J. Protozool. 6, 282–284 (1959)
Aronson, N. N., Jr., deDuve, C.: Digestive activity of lysosomes. II. The digestion of macromolecular carbohydrates by extracts of rat liver lysosomes. J. biol. Chem. 243, 4564–4573 (1968)
Bauduin, H., Colin, M., Dumont, J. E.: Energy sources for protein synthesis and enzymatic secretion in rat pancreas in vitro. Biochim. biophys. Acta (Amst.) 174, 722–733 (1969)
Bessey, O. A., Lowry, O. H., Brock, M. J.: A method for the rapid determination of alkaline phosphatase with five cubic millimeters of serum. J. biol. Chem. 164, 321–329 (1946)
Biely, P., Farkas, V., Bauer, S.: Secretion of β-glucanase by Saccharomyces cerevisiae protoplasts. FEBS Letters 23, 153–156 (1972)
Dingle, J. T.: The extracellular secretion of lysosomal enzymes. In: Lysosomes in biology and pathology, Vol. 2 (J. T. Dingle, H. B. Fell, eds.), pp. 421–436. London: North-Holland 1969
Gomori, G.: Preparation of buffers for use in enzyme studies. In: Methods in enzymology, Vol. 1 (S. P. Colowick, N. O. Kaplan, eds.), pp. 138–146. New York Academic Press 1955
Hartree, E. F.: Determination of protein: A modification of the Lowry method that gives a linear photometric response. Analyt. Biochem. 48, 422–427 (1972)
Jamieson, J. D., Palade, G. E.: Condensing vacuole conversion and zymogen granule discharge in pancreatic exocrine cells: Metabolic studies. J. Cell Biol. 48, 503–522 (1971)
Lampen, J. O.: Secretion of enzymes by micro-organisms. In: Function and structure in microorganisms, 15th Symp. Soc. Gen. Microbiol. (M. R. Pollock, M. A. Richmond, eds.), pp. 115–133. England: Cambridge University Press 1964
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with the Folin phenol reagent. J. biol. Chem. 193, 265–275 (1951)
Muller, M.: Digestion. In: Protozoa, Vol. 1 (G. W. Kidder, ed.), pp. 351–377, New York: Academic Press 1967
Muller, M.: Secretion of acid hydrolases and its intracellular source in Tetrahymena puriformis. J. Cell Biol. 52, 478–487 (1972)
Patni, N. J., Aaronson, S.: Partial characterization of the intra-and extracellular acid phosphatase of an alga, Ochromonas danica. J. gen. Microbiol. 83, 9–20 (1974)
Pollock, M. R.: Exoenzymes. In: The bacteria, Vol. 4 (I. C. Gunsalus, R. Y. Stanier, eds.), pp. 121–178. New York: Academic Press 1962
Reithel, F. J.: Phosphoglucose isomerase. II. Mammary gland. In: Methods in enzymology, Vol. 9 (S. P. Colowick, N. O. Kaplan, eds.), pp. 565–568 New York: Academic Press 1966
Van Rijn, H. J. M., Boer, P., Steyn-Parve, E. P.: Biosynthesis of acid phosphatase of bakers yeast. Factors influencing its production by protoplasts and characterization of the secreted enzyme. Biochim. biophys. Acta (Amst.) 268, 431–441 (1972)
Rogers, H. J.: The dissimilation of high molecular weight substances. In: The bacteria, Vol 2 (I. C. Gunsalus, R. Y. Stanier, eds.), pp. 257–302, New York: Academic Press 1961
Rothstein, T. L., Blum, J. J.: Lysosomal physiology in Tetrahymena. I. Effect of glucose, acetate, pyruvate and carmine on intracellular content and extracellular release of three acid hydrolases. J. Cell Biol. 57, 630–641 (1973)
Rothstein, T. L., Blum, J. J.: Lysosomal physiology in Tetrahymena. II. Effect of culture age and temperature on the extracellular release of three acid hydrolases. J. Protozool. 21, 163–168 (1974)
Stewart, J. E., Schotz, M. C.: Studies on release of lipoprotein lipase activity from fat cells. J. biol. Chem. 246, 5749–5753 (1971)
Varner, J. E., Mense, R. M.: Characteristics of the process of enzyme release from secretory plant cells. Plant Physiol. 49, 187–189 (1972)
Zurier, R. B., Hoffstein, S., Weissmann, G.: Mechanisms of lysosomal enzyme release from human leukocytes. I. Effect of cyclic nucleotides and colchicine. J. Cell Biol. 58, 27–41 (1973)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Meyer, D.H. Secretion of β-glucosidase by Ochromonas danica . Arch. Microbiol. 109, 263–270 (1976). https://doi.org/10.1007/BF00446637
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00446637